You are here: Urology Textbook > Surgery (procedures) > Prostate brachytherapy
Prostate Brachytherapy: Surgical Technique and Complications
Indications for Prostate Brachytherapy
Brachytherapy is a treatment option for localized prostate carcinoma for patients in the low and partly in the intermediate risk group. The EAU guideline limits the indications as follows: clinical stage T1–2a, a maximum of 50% affected biopsy cylinder with Gleason sum 6 (ISUP grade 1) or a maximum of 33% affected biopsy cylinder with Gleason 3+4 (ISUP grade 2), a PSA concentration below 10 ng/ml, a prostate volume below 50 ml, and no relevant LUTS (IPSS below 12 and maximum uroflow above 15 ml/s). Some authors use LDR brachytherapy also in unfavorable intermediate-risk and high-risk prostate cancer; see section prostate cancer/brachytherapy.
Contraindications
Prostate volume over 60 ml, significant LUTS, chronic inflammatory bowel disease, prostatic fossa after TURP, and previous pelvic irradiation of other organs.
 |
Preoperative Patient Preparations
- Reduction of prostate volume: if the prostate volume is too large (over 50 ml), a volume reduction of 30–40% is possible with antiandrogenic therapy over four months.
- Bowel preparation: enema the night before surgery.
- Perioperative antibiotic prophylaxis: e.g., 2nd generation cephalosporin.
- Anesthesia: general or spinal anesthesia.
- Patient positioning: lithotomy position, skin disinfection, and draping of perineum. Cystoscopy and transurethral catheter. Elevate the scrotum with an adhesive bandage.
Surgical Technique of Prostate Brachytherapy
Planning of the Seed Positions:
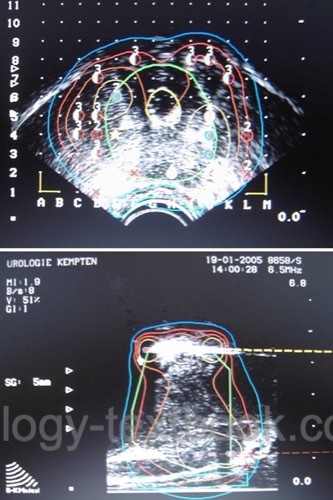
Insert a transrectal ultrasound probe into the rectum and fix it to a probe holder. A template is attached to the probe holder and projected onto the ultrasound image; this facilitates exact perineal targeting. Drape the ultrasound probe and holder with sterile blankets.
The prostate is imaged horizontally in 5 mm increments, starting at the bladder neck and moving toward the apex. Import the images into the planning software and mark the prostate borders, urethra, and rectum. The planning software calculates the prostate volume and a seed implantation plan (dosimetry) with isodose lines.
The target volume usually includes a safety distance from the prostate capsule of 3–8 mm, depending on the oncological risk and tumor localization. The prescription dose for 125iodine is 145 Gy. The software considers the following variables: V100 (fraction of target volume in % with at least 100% of prescription dose), urethral and rectal V125 or V150 (excessive radiation exposure of urethra or rectum), V200 (fraction of target volume with 200% of prescription dose). D100 is another critical parameter, also called the minimum dose index of the prostate: Dose covering 100% of the prostate volume.
Seeds Implantation:
The iodine seeds are implanted perineally according to the suggestion of the planning software. Using seed strands speeds up implantation and prevents the migration of individual seeds. Mark the position of the implanted seeds in the computer application immediately after implantation. The software recalculates the positions of the remaining seeds (intraoperative dosimetry) [fig. prostate brachytherapy]. A pelvic X-ray at the end of surgery documents the position and number of implanted seeds.
Postoperative Care
General Measures:
Early mobilization. The catheter stays until hematuria resolves, usually less than one day. Check residual volume after voiding. Prophylactic administration of an alpha blocker reduces LUTS after brachytherapy.
Follow-up Dosimetry:
Within the first six weeks after seed implantation, a pelvic CT scan is done to record the location of the seed for follow-up dosimetry. The D90 should be above 140 Gy, meaning 90% of the prostate volume receives a radiation dose of at least 140 Gy. In case of a relevant underdosage of parts of the prostate, additional seed implantation is possible.
PSA bounce:
An elevated PSA concentration after brachytherapy is not necessarily a progression of the underlying disease but may be caused by the radiation effect on the prostate tissue. PSA bounce occurs in 44% of men and may persist for up to three years until the end of the radiation effect (Critz et al., 2003).
Complications
The complications of brachytherapy are dose-dependent. The lack of intraoperative dosimetry or additional EBRT due to insufficient postoperative dosimetry increases the rate of side effects (Sarosdy et al., 2004).
Obstructive and irritative voiding symptoms:
Obstructive symptoms (residual urine, urinary retention) are caused by prostate edema after seed implantation and are usually only temporary. Longer-term obstruction results from bladder neck or urethral stricture. Irritative voiding symptoms (pollakiuria, dysuria, nycturia, urge) result from trauma, radiogenic damage to the bladder and urethra, and obstruction. Use alpha-blockers to treat LUTS. A suprapubic catheter is necessary in patients with urinary retention.
TURP for persistent urinary retention:
Due to the loss of radiation effect and the risk of incontinence, TURP should be avoided immediately after seed implantation. Spontaneous healing of urinary retention is likely after the resolution of prostatic edema. In patients with persistent urinary retention, placement of a suprapubic catheter and awaiting prostatic atrophy due to the radiation effect can avoid TURP in most of the patients.
Rectal side effects:
Radiation proctitis may cause stool urge, mucus secretion, or hematochezia. Rectal fistulas occur extremely rarely (less than 1%), but the therapeutic consequences are serious: urinary diversion and colostomy may be necessary temporarily.
Erectile Dysfunction:
50% of preoperatively potent men will experience significant deterioration of erectile function within three years.
| Robotic-assisted laparoscopic prostatectomy | Index | Brachytherapy of prostate cancer |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Critz u.a. 2003 CRITZ, F. A. ; WILLIAMS,
W. H. ; LEVINSON, et a.:
Prostate specific antigen bounce after simultaneous irradiation for
prostate cancer: the relationship to patient age.
In: J Urol
170 (2003), Nr. 5, S. 1864–7
Deger u.a. 2001 DEGER, S. ; BOHMER, D. ;
ROIGAS, et a.:
[Brachytherapy of local prostatic carcinoma].
In: Urologe A
40 (2001), Nr. 3, S. 181–4
Langley und Laing 2002 LANGLEY, S. E. ; LAING,
R.:
Prostate brachytherapy has come of age: a review of the technique and
results.
In: BJU Int
89 (2002), Nr. 3, S. 241–9
Nag u.a. 1999 NAG, S. ; BEYER, D. ;
FRIEDLAND, et a.:
American Brachytherapy Society (ABS) recommendations for
transperineal permanent brachytherapy of prostate cancer.
In: Int J Radiat Oncol Biol Phys
44 (1999), Nr. 4, S. 789–99
Sarosdy 2004 SAROSDY, M. F.:
Urinary and rectal complications of contemporary permanent
transperineal brachytherapy for prostate carcinoma with or without external
beam radiation therapy.
In: Cancer
101 (2004), Nr. 4, S. 754–60
 Deutsche Version: Technik und Komplikationen der Prostata-Brachytherapie
Deutsche Version: Technik und Komplikationen der Prostata-Brachytherapie
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.