You are here: Urology Textbook > Testes > Germ cell tumors
Germ Cell Tumor of the Testis: TNM-Stages and Diagnosis
Definition
Germ cell tumors are the most common malignancy of the young male, arising from the germinative epithelium of the testis or, less commonly, from scattered embryonic germ cells as extragonadal germ cell tumors (see section extragonadal germ cell tumors).
Epidemiology of Testicular Germ Cell Tumors
Overall, testicular tumor is rare, accounting for 1–2% of all tumor cases. The lifetime risk is approximately 0.4%. Between the ages of 20 and 44, malignant testicular tumor is the most common cancer in men (proportion 25%).
- Incidence: 6.4 per 100.000 in the USA, 10 per 100.000 in Germany, with an increasing trend. The mortality in Germany is 0.4/100.000 (RKI, 2021).
- Testicular tumors in children are uncommon (0.2 per 100.000 in Europe), mostly benign, and usually unrelated to germ cell neoplasia in situ (see pathology), e.g., teratoma, yolk sac tumor, or stromal tumors.
Pathogenesis and Risk Factors of Germ Cell Tumors
Pathogenesis:
A germ cell tumor results from defective maturation of the primordial germ cell and associated uninhibited cell division and proliferation. The transformed germ cells are initially localized basally in the seminiferous tubules and repress spermatogenesis. These lesions are called germ cell neoplasia in situ (GCNIS). GCNIS is dormant, and further proliferation and formation of seminomas and nonseminomas occur after puberty. Molecular causes of tumor progression have been partially identified: Overexpression of p53, detection of excess copies of isochromosome i(12p), loss of expression of c-Kit, and deregulation of the cell cycle at the G1/S checkpoint.
Germ cell tumors without GCNIS:
Germ cell tumors without GCNIS are rare and typical for tumors in prepuberty (teratoma, yolk sac tumor) and in males of older age (spermatocytic seminoma).
Risk factors for Germ Cell Tumors:
Congenital characteristics (polygenic pathogenesis) and environmental influences promote malignant degeneration of the germinal epithelium.
- Cryptorchidism: the tumor risk is 10–20 times greater than in the normally descended testis. The higher the position of the undescended testis, the greater the risk of malignancy. Early orchidopexy reduces the tumor risk and allows clinical controls for early detection.
- Family history: double relative risk with an affected father, fourfold risk with an affected brother, and up to 20-fold risk with an affected twin brother (Kharazmi et al., 2015). Data support a polygenic pathogenesis model in which multiple genes with low penetrance cause the disease.
- Contralateral germ cell tumor: develops within 15 years in 2%; this corresponds to a 12-fold relative risk (Travis et al., 2010).
- Ethnicity: there is a 4-fold increased relative risk for white men in the USA compared to black men.
- Environmental toxins: the increasing incidence in industrialized countries argues for environmental toxins, which increase the risk for germ cell tumors and other diseases (infertility, cryptorchidism, hypospadias, premature births). Numerous substances (endocrine disruptors) in plastics and pesticides are discussed as causal substances (DeToni et al., 2019).
- Further risk factors are testicular microlithiasis, male infertility, diethylstilbestrol in pregnancy, cannabis use (controversial), and mumps orchitis (controversial).
Pathology
Pathological Classification of Germ Cell Tumors:
The WHO classification distinguishes between germ cell tumors originating from a non-invasive germ cell neoplasia (GCNIS) and germ cell tumors unrelated to GCNIS (Moch et al., 2016).
- Germ cell tumors originating from GCNIS
- Germ cell neoplasia in-situ (GCNIS)
- Seminoma
- Nonseminoma
- Embryonal carcinoma
- Yolk sac tumor of postpubertal type
- Teratoma
- Teratoma with malignant somatic transformation
- Trophoblastic tumors: choriocarcinoma, placental site trophoblastic tumor and others.
- Mixed germ cell tumors
- Germ cell tumors unrelated to GCNIS
- Spermatocytic tumor
- Teratomas of prepubertal type, including dermoid cyst, epidermoid cyst.
- Yolk sac tumors of prepubertal type
- Sex cord-stromal tumors
- Leydig cell tumor
- Sertoli cell tumor
- Granulosa cell tumor
- Mixed and poor differentiated forms, including androblastoma
- Tumors containing both germ cell and sex cord components: Gonadoblastoma
| Do you want to see the illustration? Please support this website with a Steady membership. In return, you will get access to all images and eliminate the advertisements. Please note: some medical illustrations in urology can be disturbing, shocking, or disgusting for non-specialists. Click here for more information. |
| Do you want to see the illustration? Please support this website with a Steady membership. In return, you will get access to all images and eliminate the advertisements. Please note: some medical illustrations in urology can be disturbing, shocking, or disgusting for non-specialists. Click here for more information. |
| Do you want to see the illustration? Please support this website with a Steady membership. In return, you will get access to all images and eliminate the advertisements. Please note: some medical illustrations in urology can be disturbing, shocking, or disgusting for non-specialists. Click here for more information. |
Germ Cell Neoplasia in situ (GCNIS):
Older terms for GCNIS are carcinoma in situ (CIS) or testicular intraepithelial neoplasia (TIN). GCNIS is usually disseminated throughout the testis; a small amount of testicular parenchyma (3 mm diameter or needle biopsy) is sufficient for detection. When GCNIS is present, progression to an invasive germ cell tumor develops in 50% within five years. Risk factors for GCNIS in the contralateral tumor-free testis are testicular atrophy, cryptorchidism, and age less than 30 years.
Seminoma
Seminomas are whitish, well-demarcated, nonencapsulated tumors with occasional hemorrhage and necrosis [fig. macroscopic findings of seminoma]. 40–55% of testicular germ cell tumors. Uncommon in childhood, typically between 30–50 years of age, the second peak in patients over 60. HCG production is present in 10–20%, and the release of AFP excludes seminoma. Treatment recommendations differ between seminoma and nonseminoma; see sections on treatment of seminomas and treatment of nonseminomas.
Classic seminoma:
80–85% of seminomas. 10–15% are associated with syncytiotrophoblastic elements and with HCG production. Classic seminoma grows slowly; retroperitoneal recurrence may occur up to 2–10 years after therapy.
Anaplastic seminoma:
5–10% of seminomas, but responsible for 30% of seminoma deaths. Many criteria of high malignancy are present in histopathology: mitotic index, nuclear pleomorphism, invasion, and metastasis. However, compared with the same tumor stage, the prognosis is expected to be the same as for classic seminoma.
Spermatocytic Tumor:
1% of germ cell tumors, mostly in men over 50 years of age. Formerly classified with seminomas, spermatocytic tumor is now considered a separate entity. The tumor cells resemble maturing spermatogonia and show no relation to germ cell neoplasia in situ. The metastatic potential is low, and the prognosis is excellent.
Rarely sarcomatous portions are detected in spermatocytic tumors, which is a sign of a highly aggressive tumor with a poor prognosis; multimodal therapy is necessary (RLA, chemotherapy, radiation therapy).
Nonseminoma
40–60% of germ cell tumors are nonseminomas consisting of tissue types listed below, which occur singly or in a wide variety of combinations. Treatment recommendations differ between seminoma and nonseminoma; see sections on treatment of seminomas and treatment of nonseminomas.
Embryonal carcinoma (30–50%):
Grayish-white fleshy tumor with hemorrhages, cysts, and necrosis [fig. macroscopic findings of embryonal carcinoma]. Microscopy shows an epithelial character. Tumor markers AFP and HCG may be elevated.
Choriocarcinoma (5–10%):
Choriocarcinoma consists of placenta-like structures (syncytoblasts and cytotrophoblasts). Advanced metastasis with a small primary tumor is typical. Tumor marker HCG is typically highly elevated, and AFP facultatively positive.
Teratoma (30–50%):
Teratoma is further classified into mature teratoma, immature teratoma, or teratoma with malignant somatic transformation. Teratomas typically present with normal tumor markers, but sometimes AFP may be mildly elevated.
In mature teratomas, well-differentiated structures of the three germ layers (endoderm, mesoderm, and ectoderm) are found. Immature teratomas resemble the fetal developmental stages of the three germ layers. In rare cases, teratoma undergoes malignant somatic transformation, resulting in sarcomas, primary neuroectodermal tumors (PNET), or carcinomas. Teratomas with malignant somatic transformation can metastasize and respond poorly to chemotherapy (Giannatempo et al., 2016).
Yolk sac tumors (5–10%):
Yolk sac tumors are the predominant GCT in children under three years. AFP is in contrast to HGC elevated. Outdated terms: entodermal sinus tumor, infantile embryonal carcinoma, or orchioblastoma.
Mixed germ cell tumors (10–30%):
Mixed germ cell tumors contain various components of seminoma and nonseminoma. Therapy is based on the guidelines for nonseminomas. See also fig. macroscopic findings of mixed germ cell tumor.
Metastasis of Germ Cell Tumors
Lymphogenic metastasis:
Germ cell tumors most likely metastasize first to the retroperitoneal lymph nodes. Right-sided tumors are more prone than left-sided tumors to metastasize to the contralateral retroperitoneum. Limited retroperitoneal lymph node metastasis can be cured by retroperitoneal lymphadenectomy. As they progress, germ cell tumors metastasize further cranially along the lymphatic pathways into the retrocrural and supradiaphragmatic lymph nodes.
The testicular decensus explains the almost invariable lymphatic drainage to the retroperitoneal lymph nodes. An exception is the altered lymphatic drainage after trauma or previous surgery with additional drainage into the pelvic and inguinal lymph nodes.
Risk factors for lymph node metastasis are invasion of lymphatic vessels of the primary tumor. In addition, in seminoma, the size of the primary tumor (>4 cm) and invasion of the rete testis are risk factors for retroperitoneal lymph node metastasis.
Hematogenous metastasis:
Hematogenous metastases occur significantly later than lymphogenous metastases. The probability of pre-existing hematogenous metastasis at tumor stage pN0 is 5–8%. Risk factors for hematogenous metastasis are vascular invasion of the primary tumor.
TNM Tumor Staging of Germ Cell Tumors
T:
Primary tumor.
- T0: no evidence of primary tumor.
- Tis: germ cell neoplasia in situ (GCNIS)
- T1: tumor confined to testis and epididymis, no vascular or lymphatic invasion.
- T2: tumor confined to the testis and epididymis with vascular or lymphatic invasion or infiltration of the tunica vaginalis.
- T3: invasion of the spermatic cord.
- T4: invasion of the scrotum.
N:
Regional lymph nodes.
- N0: no regional lymph node metastasis.
- N1: enlarged lymph nodes ≤ 2 cm (cN1), maximum five lymph node metastases less than 2 cm in diameter in the specimen (pN1).
- N2: enlarged lymph nodes 2–5 cm (cN2), or more than five lymph node metastases less than 2 cm in diameter in the specimen (pN2) or extranodal extension of lymph node metastasis (pN2).
- N3: lymph nodes mass larger than 5 cm
M:
Distant metastasis.
- M0: no distant metastasis.
- M1: distant metastasis.
- M1a: nonregional nodal or pulmonary metastasis.
- M1b: further locations than M1a
S:
Serum tumor markers. The lowest value (nadir) after orchiectomy applies.
- S0: all tumor markers within normal limits.
- S1:
- AFP <1000 ng/ml and
- HCG <5000 IU/l and
- LDH <1.5 times the upper limit of the normal range
- S2:
- AFP 1000–10000 ng/ml or
- HCG 5000–50000 IU/l or
- LDH 1,5–10 times the upper limit of the normal range
- S3:
- AFP >10000 ng/ml or
- HCG >50000 IU/l or
- LDH >10 times the upper limit of the normal range
UICC Tumor Stages
Stage 0:
Germ cell neoplasia in situ (GCNIS), N0, M0, S0
Stadium I:
No visible metastases
- IA: pT1, N0, M0, S0
- IB: pT2–4, N0, M0, S0
- IS: pT1–4, N0, M0, S1–3
Stadium II:
Regional retroperitoneal lymph node metastases
- IIA: pT1–4, N1, M0, S0–1
- IIB: pT1–4, N2, M0, S0–1
- IIC: pT1–4, N3, M0, S0–1
Stadium III:
Advanced lymph node metastasis or distant metastases
- IIIA: pT1–4, N1–3, M1a, S0–1
- IIIB: pT1–4, N1–3, M0–1a, S2
- IIIC: pT1–4, N1–3, M0–1b, S3
Prognostic Risk Groups of Metastatic Germ Cell Tumors
The prognostic risk groups of metastatic GCT are classified according to the guidelines of the International Germ Cell Cancer Collaborative Group (IGCCCG) [see table below]. The grouping determines the prognosis and the number of recommended chemotherapy cycles for treatment. The tumor markers' lowest value (nadir) after orchiectomy is used for grouping (Kier et al., 2017).
Good prognosis
- 90% of seminomas have a good prognosis with a five-year progression-free survival (PFS) of 82–87% and a five-year survival rate (5-YSR) of 86–93%: any tumor marker and no non-pulmonary visceral metastases and any tumor location.
- 56% of nonseminomas have a good prognosis with a five-year progression-free survival (PFS) of 89–90% and a five-year survival rate (5-YSR) of 92–95%: Tumor marker stage S0–S1 and No non-pulmonary visceral metastases primary tumor in testicular or retroperitoneal location.
Intermediate prognosis
- 10% of seminomas have an intermediate prognosis with a five-year progression-free survival (PFS) of 67% and a five-year survival rate (5-YSR) of 72%: any tumor marker and non-pulmonary visceral metastases and any tumor location.
- 28% of nonseminomas have an intermediate prognosis with a five-year progression-free survival (PFS) of 75% and a five-year survival rate (5-YSR) of 80–85%: Tumor marker stage S2 and No non-pulmonary visceral metastases primary tumor in testicular or retroperitoneal location.
Poor prognosis
- The poor prognosis does not apply to seminomas.
- 16% of nonseminomas have a poor prognosis with a five-year progression-free survival (PFS) of 41–55% and a five-year survival rate (5-YSR) of 48–64%: Tumor marker stage S3 or non-pulmonary visceral metastases or primary tumor in mediastinal location.
Signs and Symptoms of Germ Cell Tumors
Local symptoms:
Painless testicular tumor, fluoroscopy (diaphanoscopy) is not possible. In 10–50% testicular pain.
Symptoms due to hormone production:
Gynecomastia 1–5%.
Symptoms due to metastases:
10% of patients present with symptoms of metastatic germ cell tumor: back pain, dyspnea, cough, body weight loss, leg edema, or neurologic symptoms. Palpable abdominal tumor due to retroperitoneal metastases (bulky disease). Without therapy, germ cell tumors present with a very rapid disease progression; tumor doubling time in metastases is sometimes less than one month.
Diagnosis of Germ Cell Tumors
Tumor Marker
Testicular tumor markers (except LDH) are highly specific and sensitive due to a lack of physiological production in males. Even a low tumor burden of 105 cells can be detected by tumor markers long before imaging shows tumor growth. Only 10–15% of advanced germ cell tumors have normal tumor markers.
It is essential to measure the tumor markers before orchiectomy. In elevated markers, further determinations are necessary after orchiectomy to assess the declining kinetics and the lowest value (nadir). Residual tumor is likely if the half-lives of the tumor markers (see below) are longer than normal, even if the nadir is within the normal range. The level of tumor markers after orchiectomy correlates best with the prognosis of patients. Therefore, serologic staging (S0 to S3) has been introduced in the TNM classification. The lowest value (nadir) of the tumor markers after orchiectomy is used for the grouping.
Alpha-fetoprotein (AFP):
The normal value of AFP is <15 ng/ml or <10 IU/ml. The half-life is five days, see section AFP laboratory test. AFP elevation indicates nonseminomatous testicular cancer. Mature teratomas, pure seminomas, or pure choriocarcinomas do not secrete AFP.
Human chorionic gonadotropin (HCG):
The normal value of HCG in males is <5 mIU/ml. Half-life 24–36h, see HCG laboratory test. In germ cell tumors, HCG is produced in both seminoma and nonseminoma. Values above 500 mIU/ml speak against a pure seminoma. HCG may be highly elevated in choriocarcinoma; this correlates with the (disseminated) tumor mass.
Lactate dehydrogenase (LDH):
The normal value of LDH is 60–120 U/l, see LDH laboratory test. LDH is a ubiquitous intracellular enzyme that is not specific for a germ cell tumor. LDH is useful to monitor treatment success and to judge the prognosis of advanced tumors, as the enzyme correlates very well with the tumor burden.
Placental alkaline phosphatase (PLAP):
HCG-negative seminomas sometimes express PLAP, but false-positive results are possible in smokers. PLAP is not a standard diagnostic test.
M371-Test:
The M371-Test uses quantitative PCR to detect microRNA miR-371a-3p in serum. The test shows a significantly higher sensitivity (90%) and specificity (94%) than all other available markers; all germ cell tumors express the microRNA except teratomas (Dieckmann et al., 2019). The M371 assay is not (yet) a standard diagnostic test.
Ultrasound Imaging of the Testes
Testicular tumors present as hypoechoic or mixed echoic tumors in the testicular parenchyma. The partially cystic or highly inhomogeneous lesions show an increased blood flow [fig. ultrasound imaging of germ cell tumors]. Ultrasound imaging of the contralateral testis is obligatory to exclude bilateral testicular tumors and to assess the risk for GCNIS (testicular volume? testicular microlithiasis?).
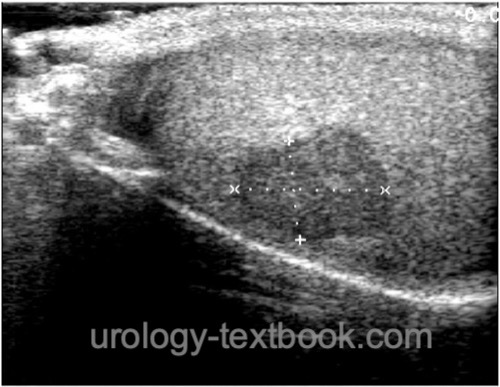 |
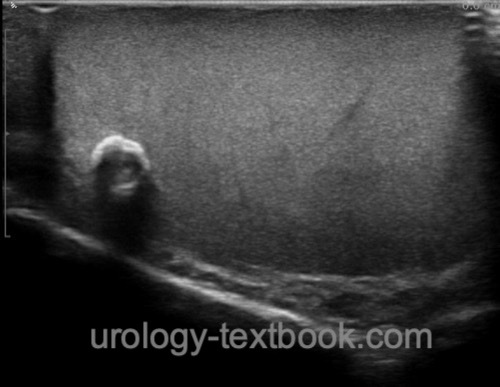 |
| Do you want to see the illustration? Please support this website with a Steady membership. In return, you will get access to all images and eliminate the advertisements. Please note: some medical illustrations in urology can be disturbing, shocking, or disgusting for non-specialists. Click here for more information. |
Staging of Germ Cell Tumors
CT scan of abdomen and pelvis:
CT scanning is the imaging technique of first choice for detecting retroperitoneal lymph node metastases or liver metastases. Alternatively, an MRI of the abdomen is an option in children or patients with contrast medium allergies (see below).
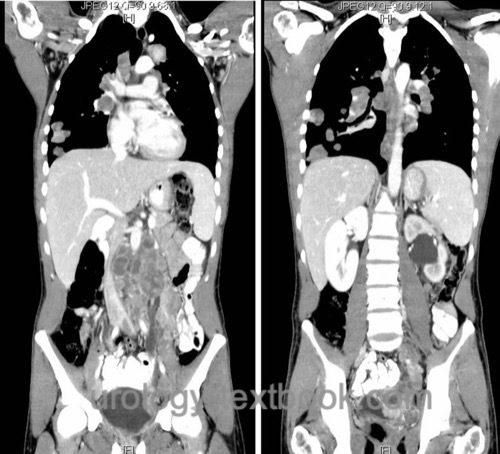 |
CT scan of the chest:
A chest CT is the imaging technique of first choice for detecting lung metastases or mediastinal lymph node metastases. CT of the abdomen and chest are performed simultaneously [fig. CT scan of advanced germ cell tumor].
Fertility Counselling
Patients with current or possible future desires for children must be offered the option of cryopreservation before orchiectomy, and fertility should be determined beforehand: semen analysis, testosterone, LH, and FSH. In patients with azoospermia, (bilateral) testicular sperm extraction can be performed during orchiectomy. All public health insurances in Germany lately cover the cost of precautionary cryopreservation.
Optional Diagnostic Tests
Abdominal MRI:
Abdominal MRI has a diagnostic accuracy comparable to an abdominal CT scan. Advantages are the lack of radiation exposure and the better-tolerated contrast agent. Disadvantages include longer examination time, lack of simultaneous chest imaging, and higher cost. The guidelines do not recommend routine use of MRI in staging. Recognized indications for MRI of the abdomen include children and intolerance of iodinated contrast material.
Testicular MRI:
Testicular MRI is an option for imaging suspicious findings in ultrasound if surgical exposure is not deemed necessary (rare indication!).
Abdominal ultrasound imaging:
Abdominal Ultrasound imaging provides a quick diagnosis of advanced disease [fig. US of retroperitoneal metastasis]. Abdominal CT is more sensitive and cannot be replaced by sonography.
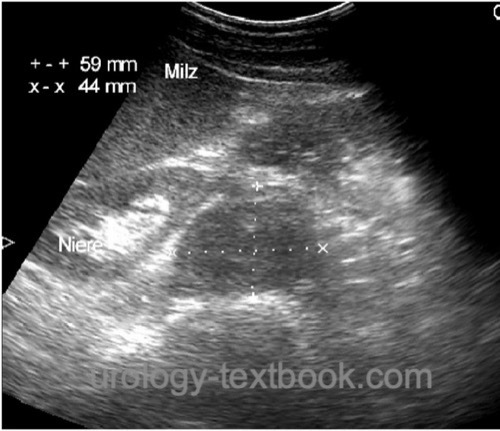 |
Cranial MRI or CT:
Cranial imaging is necessary for patients with advanced disease or symptoms suspicious of brain metastases.
Bone scintigraphy:
Perform bone scintigraphy in patients with clinical suspicion of bone metastases.
PET:
PET imaging has no role in the primary staging of germ cell tumors before first-line therapy. PET is indicated in the diagnosis of residual lymph node enlargement after chemotherapy or radiotherapy. An increased metabolic activity of the tracer (FDG) can differentiate between scar tissue and tumor tissue. Another indication for PET is an increase in testicular tumor markers during follow-up.
Testicular biopsy:
An inguinal approach to the testis with tumor enucleation is an option to prevent orchiectomy in patients with a small tumor with unclear dignity on ultrasound imaging or MRI. The lesion is examined by a frozen section. Benign tumors need no safety margin, and orchiectomy is unnecessary. If a malignant germ cell tumor is detected by frozen section, radical orchiectomy is the standard procedure. Organ-sparing surgery may be considered for small malignant lesions after complete resection of the tumor, especially in cases of bilateral manifestation or patients with a single testis. Since there is a high risk of concomitant GCNIS, adjuvant local radiotherapy of the testis with 16–20 Gy should be performed.
| Do you want to see the illustration? Please support this website with a Steady membership. In return, you will get access to all images and eliminate the advertisements. Please note: some medical illustrations in urology can be disturbing, shocking, or disgusting for non-specialists. Click here for more information. |
Contralateral testicular biopsy:
The risk of contralateral GCNIS is between 5–10%. Risk factors for contralateral GCNIS are a testicular volume of less than 12 ml, a history of cryptorchidism, and an age under 30 years, increasing the risk to ≥34%. The options of contralateral testicular biopsy should be discussed with risk patients.
If cisplatin-based chemotherapy is already foreseeable at the time of orchiectomy, a contralateral testicular biopsy should not be performed. Chemotherapy cures GCNIS in more than 60% of cases. If risk factors for GCNIS are present, contralateral testicular biopsy should be performed two years after completion of therapy for the primary tumor, if necessary.
Screening for Germ Cell Tumor
A specific (physician) screening program for testicular cancer is not recommended (PDQ, 2021). Arguments against a screening program are the low incidence of germ cell tumors and the excellent treatment results even in advanced tumor stages. The lack of benefit or theoretically low benefit of screening would be associated with a high rate of false positive results, anxiety, and harm from unnecessary diagnostic procedures such as testicular biopsies. The German Urology Society recommends monthly self-examination of men between 15 and 45 years of age and calls for initial medical guidance, but health insurance currently rejects this.
Differential Diagnosis
See differential diagnosis of testicular tumors.
| Sertoli-cell tumor | Index | Treatment of seminoma |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Dieckmann, K.-P.; Radtke, A.; Geczi, L.; Matthies,
C.; Anheuser, P.; Eckardt, U.; Sommer, J.; Zengerling, F.; Trenti, E.;
Pichler, R.; Belz, H.; Zastrow, S.; Winter, A.; Melchior, S.; Hammel, J.;
Kranz, J.; Bolten, M.; Krege, S.; Haben, B.; Loidl, W.; Ruf, C. G.;
Heinzelbecker, J.; Heidenreich, A.; Cremers, J. F.; Oing, C.; Hermanns,
T.; Fankhauser, C. D.; Gillessen, S.; Reichegger, H.; Cathomas, R.;
Pichler, M.; Hentrich, M.; Eredics, K.; Lorch, A.; Wülfing, C.; Peine, S.;
Wosniok, W.; Bokemeyer, C. & Belge, G.
Serum Levels of
MicroRNA-371a-3p (M371 Test) as a New Biomarker of Testicular Germ Cell
Tumors: Results of a Prospective Multicentric Study.
Journal of
clinical oncology, 2019, JCO1801480
Albers, P.; Albrecht, W.; Algaba, F.; Bokemeyer,
C.; Cohn-Cedermark, G.; Fizazi, K.; Horwich, A.; Laguna, M.; Nicolai, N. &
Oldenburg, J.
EAU Guidelines: Testicular Cancer
. https://uroweb.org/guidelines/testicular-cancer/
DGU, DKG, AWMF, and L. Onkologie, “S3-Leitlinie Diagnostik, Therapie und Nachsorge der Keimzelltumoren des Hodens. Langversion 1.1.” [Online]. Available: https://www.leitlinienprogramm-onkologie.de/leitlinien/hodentumoren/
A. Stephenson, E. B. Bass, and B. R. Bixler, “Diagnosis and Treatment of Early-Stage Testicular Cancer: AUA Guideline.” [Online]. Available: https://www.auanet.org/guidelines-and-quality/guidelines/testicular-cancer-guideline
Kharazmi E., Hemminki K., Pukkala E., Sundquist K., Tryggvadottir L., Tretli S., Olsen J.H., and Fallah M. Cancer risk in relatives of testicular cancer patients by histology type and age at diagnosis: a joint study from five nordic countries, Eur Urol 2015, 68(2):283-89.
S. Krege, J. Beyer, R. Souchon, P. Albers, and EGCCCG-Members.
European consensus conference on diagnosis and treatment of germ cell
cancer: a report of the second meeting of the european germ cell cancer
consensus group (egcccg): part i.
Eur Urol, 53 (3): 478–496, Mar
2008a.
S. Krege, J. Beyer, R. Souchon, P. Albers, and EGCCCG-Members.
European consensus conference on diagnosis and treatment of germ cell
cancer: a report of the second meeting of the european germ cell cancer
consensus group (egcccg): part ii.
Eur Urol, 53 (3): 497–513, Mar
2008b.
Robert-Koch-Institut
Krebs in Deutschland 2017/2018
2021 https://www.krebsdaten.de/
W. Rodprasert, J. Toppari, and H. E. Virtanen, “Endocrine Disrupting Chemicals and Reproductive Health in Boys and Men.,” Front Endocrinol., vol. 12, p. 706532, 2021.
 Deutsche Version: Epidemiologie und Ätiologie von Hodentumoren und Pathologie und TNM Tumorstadien von Hodentumoren
Deutsche Version: Epidemiologie und Ätiologie von Hodentumoren und Pathologie und TNM Tumorstadien von Hodentumoren
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.