You are here: Urology Textbook > Testes > Male infertility
Male Infertility
Definitions and Epidemiology
Infertility is a variably defined condition with correspondingly varying epidemiological data. Most commonly, infertility is defined as one year of unwanted non-conception despite regular unprotected intercourse (EAU guidelines). The old distinction between sterility (impossibility of fathering a child) and infertility (impossibility of carrying a child to term) no longer exists in modern literature, both terms are used synonymously side by side. In prevalence studies, there are substantial differences in the duration of unwanted non-conception (1–2 years vs. lifelong), data collection (questionnaire vs. prospective), outcome goals (pregnancy vs. live birth), relationship status, and exclusion criteria (Gurunath et al., 2011). The following definitions take the duration of unwanted non-conception into account:
- Subfertility: unwanted non-conception after six menstrual cycles despite regular unprotected sexual intercourse. The prevalence is approximately 20%. Half of these couples will achieve pregnancy in the subsequent six cycles.
- Infertility: the absence of pregnancy after twelve menstrual cycles despite regular unprotected sexual intercourse. The prevalence is about 10%. About half of the couples have the prospect of spontaneous conception within 36 months, the other half suffer from definitive infertility.
- Oligoasthenoteratozoospermia: OAT is a common diagnosis based on sperm analysis in subfertile or infertile men with too few, too low motility, and too many pathologically shaped spermatozoa.
- Azoospermia: no spermatozoa are found in the ejaculate.
- Sertoli cell-only syndrome: SCOS describes male patients without evidence of spermatogenesis, the pathological diagnosis is based on a testicular biopsy.
Etiology (Causes) of Infertility
In couples with infertility, male factors are identified in 30% (see below). Female causes of infertility include ovulation disorders (25%), tubal disease (20%), and uterine or peritoneal disease. Male and female factors for infertility are present in about 40%, and no causes are found in 25% of cases.
Unexplained infertility:
Unexplained infertility is present in 25% of couples. Prerequisites for clinical diagnosis are infertility (12 months of unwanted non-conception) and normal standard examinations (semen analysis, tubal patency test and ovulation examination). The prognosis is favorable, with about 50% of couples achieving pregnancy within the next 12 months and another 12% in the following year (Gelbaya et al., 2014).
Female age:
Female age is a risk factor for infertility, see table age and pregnancy rate, and a relevant factor for the success of assisted reproductive techniques.
Congenital diseases with male infertility:
About 20% of men with azoospermia have genetic or chromosomal causes, of which Klinefelter syndrome is the most common.
Chromosomal diseases:
- Klinefelter syndrome (47,XXY) leads to azoospermia and hypergonadotropic hypogonadism.
- 46XX males also have azoospermia due to missing germ cell production.
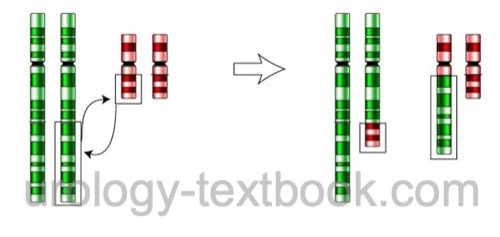
- Chromosomal balanced translocation is an exchange of chromosome arms without losing genetic material [Fig. balanced translocation]. Men with balanced translocation are usually asymptomatic but may present with impaired fertility (oligozoospermia to azoospermia) because the translocation impedes meiosis. If fertility is present, there is a high risk of zygotes with unbalanced translocation, causing a high miscarriage rate or severe disability.
 |
Genetic disorders:
- Y-linked mutations of azoospermia factor: mutations of AZFa+b lead to azoospermia, and testicular biopsy will show a Sertoli cell-only syndrome. Minor spermatogenesis is possible with mutations of AZFc; TESE is an option to enable ICSI.
- Minimal or partial androgen insensitivity syndrome.
- 5α-reductase deficiency
- Congenital adrenal hyperplasia and other defects of testosterone biosynthesis.
- CFTR mutations with bilateral aplasia of the vas deferens (CBAVD)
- Congenital hypogonadotropic hypogonadism including Kallmann syndrome
Hormonal disorders:
Patients may present with subfertility to azoospermia depending on the severity of the hormonal disorder.
- Primary, secondary, and tertiary hypogonadism
- Congenital adrenal hyperplasia
- Estrogen excess (liver cirrhosis, adrenocortical carcinoma, testicular tumors)
- Androgen intake (usually athletes)
- Hypercortisolism, hyperprolactinemia, hyperthyroidism, or hypothyroidism.
- Severe systemic diseases lead, among others, to primary hypogonadism and infertility (e.g., liver cirrhosis, chronic kidney disease or cardiovascular diseases).
Testicular failure:
Many causes lead to testicular insufficiency and infertility (with or without hypogonadism).
- Toxins and drugs: radiation therapy, chemotherapy, ketoconazole, calcium channel blockers, cimetidine, spironolactone, valproic acid, allopurinol, alpha-blockers, tricyclic antidepressants, recreational drug use.
- Cryptorchidism: the cause of germ cell damage in cryptorchidism is unclear (genetic factors, hyperthermia). Early orchidopexy (before the age of two) is essential to reduce the risk of later infertility.
- Testicular tumors: both germ cell tumors and stromal cell tumors may be the cause of infertility. Common etiologic factors for tumor induction and damage to the germinal epithelium are suspected. In addition, hormone production by tumors may lead to infertility.
- Varicocele: worsens sperm quality due to reflux of catecholamine-containing venous blood with reactive vasoconstriction, further increased testicular temperature due to increased blood flow, venous stasis, and decreased testosterone production by Leydig cells.
- Infection: Mumps orchitis, testicular abscess, or bacterial epididymitis.
- Mechanical factors: testicular torsion or testicular trauma. There is a risk of immunologic damage to the contralateral side.
- Anorchia: congenital or acquired loss of both testicles.
Posttesticular causes:
Bilateral diseases of the seminal tract lead to infertility (obstructive azoospermia).
- Genetics: bilateral aplasia of the vas deferens (CBAVD), aplasia of the epididymis or seminal vesicles due to mutations of the CFTR gene.
- Young syndrome: COPD, chronic sinusitis, and obstructive azoospermia caused by abnormal ciliary or mucosal function.
- Obstruction of the ductus ejaculatorii: due to utricle cysts, infections, prostate stones, fibrosis, spermatoceles, or ADPKD.
- Acquired obstruction: after vasectomy (5% desire surgical reanastomosis after vasectomy), hernia surgery, epididymitis.
Spermatozoa dysfunction:
- Defective flagellum: is a heterogeneous group of diseases: defective dynein, Kartagener syndrome (situs inversus, bronchiectasis, sinusitis), Usher syndrome (retinitis pigmentosa, deafness).
- Sperm maturation defects: usually due to diseases of the epididymis or after reanastomosis following vasectomy.
Immunological causes:
The blood-testicular barrier prevents antibodies from targeting spermatozoa. Antibodies against sperm are increased after testicular trauma, testicular torsion, or vasectomy. Autoimmune infertility is a common cause (10%) of male infertility. Another possibility of immunogenic infertility is female isoantibodies against spermatozoa.
Iatrogenic factors:
Chemotherapy, radiotherapy, medications (cytostatics, immunosuppressants, corticosteroids, antidepressants, metoclopramide, antibiotics, antiepileptics), vas deferens injury due to hernia repair.
Environmental toxins:
The decreasing fertility (sperm count) in industrialized countries argues for environmental toxins, which increase the risk for infertility and other diseases (cryptorchidism, hypospadias, premature births, testicular cancer). Numerous substances (endocrine disruptors) in plastics and pesticides are discussed as causal substances (DeToni et al., 2019).
Other risk factors:
Severe systemic diseases such as chronic renal failure, liver cirrhosis, and sickle cell anemia, among others. Furthermore, severe nicotine or alcohol abuse.
Sexual disorders:
Wrong timing, frequency, or practices. Premature ejaculation, retrograde ejaculation, lack of ejaculation (paraplegia).
Basic Evaluation for Male Infertility
Men with subfertility or infertility need a medical history, physical examination, testicular ultrasound imaging, and a semen analysis. Further tests are necessary for pathological findings.
Medical history:
Ask for preexisting pregnancies or abortions in previous relationships, ejaculatory dysfunction, incorrect timing or frequency of intercourse, medications, childhood illnesses (mumps orchitis), epididymitis, testicular surgery, prostate surgery, nicotine, alcohol, drugs, testosterone substitution or abuse, systemic diseases, genetic diseases, or environmental exposures (lead, ionizing radiation, chemicals).
Physical examination:
Assess the testicular volume by orchidometer or ultrasound imaging (normal 18 ml ±5 ml) and identify the vas deferens by palpation. Search for pathological findings such as varicocele, epididymitis, inguinal testis, testicular tumor, meatus stenosis, hypospadias, hair pattern, and gynecomastia.
Testicular Ultrasound Imaging:
Testicular volume? Microlithiasis? Testicular tumors? Varicocele?
Semen analysis:
Semen analysis measures volume, consistency, pH, fructose, spermatozoa count, motility, vitality, morphology, and signs of inflammation. For standard values, see the section on semen analysis. Pathological results should be confirmed after three months. Male infertility is likely if severe disorders are found (azoospermia, cryptozoospermia, complete asthenozoospermia). The significance of low-grade abnormalities is limited, and fertility is still possible. Currently, no reliable sperm function tests exist.
- Asthenozoospermia: the percentage of progressively motile spermatozoa is less than 32%.
- Cryptozoospermia: there is no detection of spermatozoa in the native semen, but (few) spermatozoa are detectable in the sediment after centrifugation.
- Azoospermia: absence of spermatozoa in the native semen and after centrifugation of the ejaculate and evaluation of the sediment.
- Necrozoospermia: a percentage of dead spermatogonia above 42%.
- Oligozoospermia: low sperm count <15 × 106 per ml or <39 × 106 total sperm count.
- Pyospermia: more than 106 leukocytes/ml of ejaculate. Detection of >103 bacteria/ml ejaculate is suggestive of significant bacteriospermia, if a urinary tract infection is not present.
- Teratozoospermia: less than 4% spermatozoa with complete normal morphology.
- Combined diagnoses: e.g., asthenoteratozoospermia, oligoasthenozoospermia, oligoasthenoteratozoospermia (OAT), oligoteratozoospermia.
Additional Tests
The following examinations may be indicated if pathological results are found in the basic evaluation (see above).
Transrectal ultrasound imaging:
Transrectal ultrasound imaging is indicated in patients with azoospermia or low semen volume. A widened seminal vesicle (>1.5 cm) or ejaculatory ducts (>2.5 mm) is suspicious for seminal tract obstruction. The absence of seminal vesicles is suggestive of CBAVD. Cysts, calcifications, and prostatic stones are also suspicious for ejaculatory duct obstruction.
Laboratory tests:
Endocrinological tests are indicated in patients with pathological semen analysis, erectile dysfunction, or decreased libido. Measure testosterone and FSH in the morning as a search test. Normal values exclude relevant hypogonadism. Laboratory tests are repeated for pathological values, and additional lab tests are ordered: SHBG, prolactin, LH, and TSH. A GnRH stimulation test is indicated in patients with decreased gonadotropins. FSH is elevated in germ cell damage (non-obstructive azoospermia) due to missing negativ feedback via inhibin. Highly elevated FSH in combination with small testes and azoospermia indicate an untreatable disorder of spermiogenesis.
DNA fragmentation index (DFI):
The DNA fragmentation index (DFI) indicates DNA damage (single and double DNA strand breaks) in the spermatozoa, which is not detectable with a normal semen analysis. DNA fragmentation results from oxidative stress and defective maturation processes in the seminal tract. Spermatozoa from the testes show significantly less DNA fragmentation. DNA fragmentation is measured with different laboratory methods, which are not comparable with each other: TUNEL (terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling), comet assay, or sperm chromatin structure assay (SCSA).
The higher the DNA fragmentation (>30%), the worse the prognosis regarding fertilization, early embryo development, implantation and abortion rate. In couples with high DNA fragmentation, spontaneous fulfillment of the child's wish is unlikely. If assisted reproduction is desired, IVF should be avoided. ICSI with spermatozoa from testis biopsy is more promising (Esteves et al., 2017).
Chromosome analysis:
Karyotyping is indicated in men with azoospermia or oligozoospermia (EAU guideline <10×106 and AUA guideline <5×106 spermatogonia). Klinefelter syndrome is the most common pathologic finding in azoospermia. Other possible findings are chromosomal translocations and rarer disorders such as 46,XX male syndrome.
Y-chromosomal mutations:
The detection of mutations on the long arm of the Y chromosome (AZF region) is indicated in men with azoospermia and severe oligozoospermia (EAU guideline <1×106 spermatogonia and AUA guideline <5×106 spermatogonia).
CFTR mutations:
The detection of CFTR mutations is indicated in infertile men without palpable vas deferens on either side (see also section CBAVD). 80% of men without detectable vas have mutations in the gene for cystic fibrosis.
Testicular biopsy:
Diagnostic biopsy is indicated in patients with azoospermia with testis of normal size and normal FSH. Testicular biopsy should always be performed with cryopreservation of testicular tissue when ICSI is planned to avoid a second procedure. See section testicular biopsy for the technique of open testicular biopsy. An alternative to open testicular biopsy is multiple percutaneous aspiration cytology of testicular tissue.
Testicular biopsy is not helpful in diseases with confirmed absence of spermatogenesis: Klinefelter syndrome, 46XX male syndrome, and AZFa+b mutations of azoospermia factor. If AZFc mutations are detected, testicular biopsy may be attempted after genetic counseling.
Diagnostic testing for female infertility:
The basic examination evaluates abnormalities of the menstrual cycle and ovulation with laboratory tests (LH, FSH, and progesterone) and ultrasound imaging. Furthermore, the patency of the tubes is checked using hysterosalpingography or laparoscopy and chromopertubation. The postcoital test and Kurzrock-Miller test (see below) examine the interaction between sperm and cervical mucus; these tests became unpopular due to a lack of standardization and reproducibility.
Postcoital test (Sims-Huhner test):
The postcoital test (Sims-Huhner test) is performed on the day of suspected ovulation and approximately 2–10 hours after sexual intercourse. Cervical mucus is collected and spread over a slide. There should be more than one progressively motile spermatozoa per visual field. IVF or ICSI is indicated if moving spermatozoa are not visible.
Kurzrock-Miller test:
The Kurzrock-Miller test evaluates the interaction between cervical mucus and sperm on a slide (in vitro). Cervical mucus and semen are spread side by side on a slide. The sperms should penetrate the area of cervical mucus and remain motile (positive test result). The test is pathological if the sperms do not penetrate the mucus or become immobile in the mucus.
Sperm penetration assay (SPA):
The SPA examines the ability of spermatozoa to penetrate a hamster egg cell. The test has lost its significance in clinical practice due to the widespread use of ICSI.
Treatment of Male Infertility
Counseling and education:
Counseling and education include information about timing and coital frequency, sexual intercourse is recommended every 2–3 days. Lubricants should not be used if possible. A healthy diet with plenty of antioxidants is recommended. Regular alcohol consumption, excessive sport, smoking, drugs, anabolic steroids and overweight (BMI over 30) should be avoided. In women, being underweight (below 15% of ideal weight) leads to secondary amenorrhea and significant disturbance of fertility.
Therapy of hormonal disorders:
Proven hormonal disorders enable a causal therapy of infertility.
Hypogonadotropic hypogonadism:
Treatment is possible with HCG stimulation or pulsatile GnRH therapy using a drug pump [see section male hypogonadism]. Direct testosterone substitution would inhibit gonadotropins (especially FSH) and is therefore detrimental.
Hyperprolactinemia:
Transsphenoidal tumor resection is indicated in patients with a macroadenoma of the pituitary gland. Hyperprolactinemia without a visible pituitary tumor is treated with bromocriptine (5–10 mg/day) and regular controls of testosterone and prolactin concentrations.
Congenital adrenal hyperplasia:
Severe infertility (azoospermia) and a testicular adrenal rest tumor are possible in congenital adrenal hyperplasia with insufficient medical therapy. Correct (sufficient) substitution of cortisone and fludrocortisone is essential for the fertility of affected men.
Treatment of Sexual Dysfunction:
Sexual dysfunction is the most common treatable cause of infertility.
Premature ejaculation:
Treatment options for premature ejaculation include topical lidocaine ointments, start-stop techniques of GV, prior masturbation, or drug therapy with serotonin reuptake inhibitors (SSRIs) such as fluoxetine, paroxetine, or dapoxetine.
Retrograde ejaculation
Treatment options are sympathomimetics such as imipramine 25–75 mg p.o. 1-1-1 or desimipramine 50 mg p.o. every other day. Alternatively, spermatozoa can be obtained from postejaculatory urine, and sperm quality can be improved by prior urinary alkalinization and increasing diuresis.
Electroejaculation
Electroejaculation is indicated in patients with paraplegia with absence of ejaculation or after (surgical) retroperitoneal nerve injury. Electrostimulation through the rectum stimulates sympathetic nerves and causes contraction of the seminal tract. The antegrade portion of the ejaculation is collected directly, and the retrograde portion is extracted with a catheter.
Therapy of inflammatory causes of male infertility:
Trials are contradictory, and treatment recommendations are poorly substantiated.
Anti-sperm antibodies:
Due to poor data for immunosuppression (glucocorticoids), ICSI is often recommended as first-line therapy.
Pyospermia:
Long-term antibiosis and frequent ejaculations may resolve chronic seminal tract infection. Suitable antibiotics are doxycycline, fluoroquinolones, erythromycin, amoxicillin, or cotrimoxazole (after culture or empirically). Some recommend a combination of antibiotic therapy with non-steroidal anti-inflammatory drugs as a therapeutic trial.
Empirical drug therapy:
A specific cause is not evident in most subfertile men. Numerous substances are empirically used for the treatment of male infertility or subfertility. The limited efficiency, costs, and possible side effects must be judged against the options of assisted reproductive technology.
Antioxidants:
Commercially available supplements with antioxidant effects contain glutathione, vitamin E, vitamin C, carotenoids, resveratrol, flavonoids and trace elements such as zinc or selenium. Several randomized trials have investigated the therapeutic effect of dietary supplements with antioxidant activity. The Cochrane meta-analysis found an increased pregnancy rate (OR 3–4) in men with subfertility (Showell et al., 2014). In principle, a healthy diet increases antioxidants as well.
Estrogen antagonists:
Tamoxifen and clomiphene lead to an increase in gonadotropins and, thus, stimulation of spermiogenesis. A meta-analysis of multiple randomized trials found a 2.4-fold improvement in pregnancy rate (Chua et al., 2013). Dosage (off-label use) of tamoxifen 10 mg 1-0-1.
Aromatase inhibitors:
Inhibition of aromatase lowers the conversion of testosterone to estradiol and is a treatment option for infertile men with low testosterone-estradiol ratios. Dosage (off-label use): testolactone 50–100 mg 1-0-1, letrozole 2.5 mg 1-0-0, or anastrozole 1 mg 1-0-0.
Recombinant FSH:
Recombinant FSH improves total sperm count and progressive motility in idiopathic male infertility in several randomized studies (Cannarella et al., 2020). Dosage (off-label use): 75–150 IU s.c. on alternate days.
Other drugs:
Conflicting data on efficacy exist for kallikrein, zinc, pentoxifylline, mast cell blockers, and L-carnitine.
Varicocelectomy for male infertility
Varicocelectomy improves semen quality in subfertile or infertile men with varicocele. The meta-analysis of Marmar (2007) found a 2.7fold increase in the pregnancy rate. Varicocelectomy reduces the DNA fragmentation of spermatozoa and improves the results of assisted fertilization (Machen et al., 2019). In men with varicocele and non-obstructive azoospermia, individual studies have demonstrated improved sperm extraction rate after varicocelectomy, with 14% sperm detection in the postoperative semen analysis (Sajadi et al., 2019). Further studies are needed. See also details on varicocele.
Obstructive azoospermia:
Before surgical therapy, intact sperm production should be confirmed with a testicular biopsy, preferably with simultaneous cryopreservation. The following surgical treatment options exist depending on the cause and location of the obstruction:
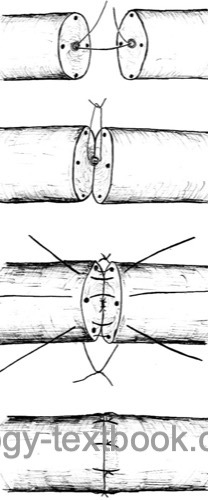
Vasovasostomy:
Vasovasostomy is indicated for obstruction of the vas deferens after vasectomy.
Microsurgical technique: excise the vasectomy scar and stricture. The patency of the proximal end (to the prostate) is tested by saline injection. Vasography during surgical therapy is not recommended since the contrast medium can induce strictures. Sperm microscopy from the distal end (from the testis) should confirm spermatozoa. A vasoepididymostomy is necessary if no spermatozoa are detectable in the distal vas.
 |
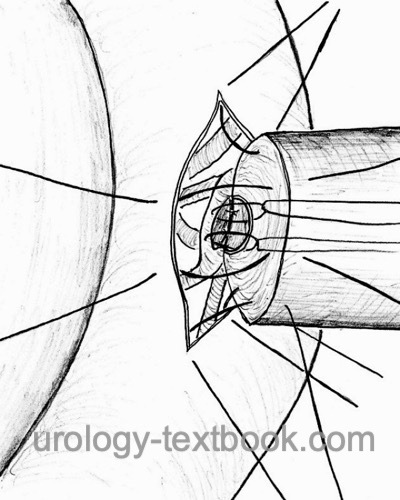
Vasoepididymostomy:
Vasoepididymostomy is indicated for obstruction of the epididymis or negative intraoperative sperm microscopy during planned vasovasostomy.
Microsurgical technique: incision of a dilated epididymal loop, microscopy of fluid, motile sperm are cryopreserved, and vasoepididymostomy (end-to-side anastomosis) is performed. Patency rates between 37 to 85% and pregnancy rates between 13 to 42% are published.
 |
Obstruction of the ejaculatory ducts:
Obstruction of the ejaculatory ducts is rare; the diagnosis is likely in patients with decreased sperm volume, azoospermia, absent fructose in seminal fluid, congested seminal vesicles, and dilated ejaculatory ducts in transrectal ultrasound imaging. The best therapeutic options are surgical sperm retrieval (TESE) and ICSI. Alternative therapy is endoscopic recanalization (TUR of the ejaculatory ducts). Spermatozoa should be detectable with a transrectal puncture of the congested seminal vesicle, and vasography of the proximal seminal ducts is done. Depending on the results of imaging, surgical therapy may be attempted; side effects include reflux of urine with epididymitis.
Assisted Reproductive Technology
Recommend the use of assisted reproductive technology if the treatment mentioned above fails or is not indicated. Assisted reproductive technology (ART) includes (surgical) sperm retrieval with processing and concentration, hormonal stimulation of the woman for controlled follicular maturation, and then various techniques of egg fertilization.
Sperm processing For insemination or IVF:
The spermatozoa are separated from the seminal plasma. After liquefaction, the semen is mixed with culture medium and centrifuged, the supernatant is discarded, and the semen is resuspended with culture medium. Excess semen can now be cryopreserved if needed. The concentration of motile sperm is possible with the swim-up method: the semen is centrifuged, the supernatant discarded, and the sperm pellet carefully coated with culture medium. Now, mobile sperm can swim into the supernatant and are aspirated.
Surgical sperm retrieval techniques:
Surgical sperm retrieval is indicated for azoospermia or relevant DNA fragmentation of spermatozoa.
Microsurgical epididymal sperm aspiration (MESA):
Microsurgical epididymal sperm aspiration (MESA) includes incision of a dilated epididymal tubule with the help of an operating microscope, aspiration of the draining fluid, and cryopreservation in aliquots to enable several ICSI cycles. MESA is done in patients during surgical refertilization (vasoepididymostomy).
Testicular sperm extraction (TESE):
Testicular sperm extraction (TESE) includes testicular tissue collection, microscopy of the processed tissue, and cryopreservation for multiple ICSI cycles. TESE is indicated for testicular or obstructive azoospermia. Collecting testicular tissue at multiple sites with microsurgical identification of dilated tubules ensures the highest success in patients with hardly existing spermatogenesis. A percutaneous biopsy technique is possible in patients with normal spermatogenesis (pTESE).
Intrauterine insemination (IUI):
Intrauterine insemination is a therapeutic option in male subfertility or pathological postcoital test. Total motile sperm count (TMSC) after semen processing should be above 5–10 million. Insemination is preceded by ovarian stimulation with gonadotropins. The delivery rate per cycle is about 8–10% (Dickey et al., 1999). Maternal age is a significant determinant of success.
%In vitro fertilization (IVF):
Several 100000 prepared mobile sperm are needed for this technique. After transvaginal ovum retrieval, fertilization is achieved in the Petri dish with prepared spermatozoa. After in vitro fertilization, zygotes are cultured until the morula stage. Depending on the woman's age and other clinical factors, 1–3 morulae are placed into the cavum uteri (embryo transfer).
Intracytoplasmic sperm injection (ICSI)}:
Intracytoplasmic sperm injection requires only a few spermatozoa. After transvaginal ovum retrieval, fertilization is achieved by single spermatozoa injection into several oocytes under a microscope. The zygotes are cultured until the morula stage. Depending on the woman's age and other clinical factors, 1–3 morulae are placed into the cavum uteri (embryo transfer). ICSI achieves pregnancy in 30% and live birth in 23% (depending on maternal age).
Complications of Assisted Reproductive Technology:
Ovulation induction with hormone stimulation of the woman can cause significant side effects: abdominal pain, injuries during retrieval, and hormone side effects. ART significantly increases the risk of a multiple pregnancy.
The risk of congenital malformations is increased, the relative risk being 1.4 (Wen et al., 2012). For this fact alone, ethical concerns exist about this technique. Preimplantation genetic diagnosis allows the selection of healthy embryos in couples with hereditary diseases. The selection of embryos and disposal of the diseased or supernumerary embryos is also highly controversial.
| Testosterone deficiency | Index | Testicular cyst |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
H. Cuppens and J. J. Cassiman, “CFTR mutations and polymorphisms in male infertility,” Int J Androl, vol. 27, no. 5, pp. 251–6, 2004.
S. C. Esteves, M. Roque, C. K. Bradley, and N. Garrido, “Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: systematic review and meta-analysis.,” Fertil Steril, vol. 108, no. 3, pp. 456–467, 2017.
Gurunath, S.; Pandian, Z.; Anderson, R. A. &
Bhattacharya, S.
Defining infertility--a systematic review of
prevalence studies.
Hum Reprod Update 2011, 17, 575-588
Gnoth, C.; Godehardt, E.; Frank-Herrmann, P.; Friol,
K.; Tigges, J. & Freundl, G.
Definition and prevalence of
subfertility and infertility.
Hum Reprod, 2005, 20,
1144-1147
G. Haidl, H. C. Schuppe, F. M. Köhn, and C. Leiber, “[Evidence-based drug therapy for male infertility],” Urologe A, vol. 47, no. 12, pp. 1555–6, 1558–60, 2008.
Hendry u.a. 2001 HENDRY, W. ; MEULEMAN, E. ;
POMEROL, J. ; PRYOR, B.:
Infertility: Urological Aspects.
In: Eur Urol
40/6 (2001)
J. L. Marmar et al., “Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis.,” Fertil Steril, vol. 88, no. 3, pp. 639–648, 2007.
J. Paick, S. H. Kim, and S. W. Kim, “Ejaculatory duct obstruction in infertile men,” BJU Int, vol. 85, no. 6, pp. 720–4, 2000.
W. Rodprasert, J. Toppari, and H. E. Virtanen, “Endocrine Disrupting Chemicals and Reproductive Health in Boys and Men.,” Front Endocrinol., vol. 12, p. 706532, 2021.
A. Salonia, S. Minhas, and C. Bettocchi, “EAU Guidelines: Sexual and Reproductive Health,” 2022. [Online]. Available: https://uroweb.org/guidelines/sexual-and-reproductive-health/.
D. Santi, A. R. M. Granata, and M. Simoni, “FSH treatment of male idiopathic infertility improves pregnancy rate: a meta-analysis.,” Endocr Connect, vol. 4, no. 3, pp. R46–R58, 2015.
P. Schlegel, M. Sigman, B. Collura, C. De Jonge, M. Eisenberg, and et al., “Diagnosis and Treatment of Infertility in Men: AUA/ ASRM Guideline.” [Online]. Available: https://www.auanet.org/guidelines-and-quality/guidelines/male-infertility
M. G. Showell, R. Mackenzie-Proctor, J. Brown, A. Yazdani, M. T. Stankiewicz, and R. J. Hart, “Antioxidants for male subfertility.,” Cochrane Database Syst Rev, vol. 12, p. CD007411, 2014.
 Deutsche Version: Ursachen und Diagnose der Infertilität des Mannes
Deutsche Version: Ursachen und Diagnose der Infertilität des Mannes