You are here: Urology Textbook > Prostate > Prostate cancer > Hormonal therapy
Prostate Cancer: Hormonal Therapy (Androgen Ablation)
- Prostate cancer: Epidemiology and etiology
- Prostate cancer: Pathology
- Prostate cancer: Signs and symptoms
- Prostate cancer: Screening
- Prostate cancer: Staging
- Prostate cancer: Treatment options
- Prostate cancer: Active surveillance
- Prostate cancer: Prostatectomy
- Prostate cancer: Radiation therapy
- Prostate cancer: Brachytherapy
- Prostate cancer: TURP and experimental treatment options
- Prostate cancer: Hormonal therapy of advanced prostate cancer
- Prostate cancer: Treatment of castration-resistant prostate cancer
Guidelines and review literature: (EAU Guidelines Prostate Cancer) (S3-Leitlinie Prostatakarzinom) (Walsh-Campbell Urology).
Advanced prostate cancer is classified into different stages depending on the response to standard hormone therapy and imaging results. The following disease stages have been defined by pivotal studies to guide the indications of modern hormone therapy:
Castration-sensitive prostate carcinoma (CSPC):
CSPC is an advanced prostate carcinoma (biochemical progression or proven metastases) with a response to androgen deprivation (see below) Depending on the detection of metastases on imaging, the stage is referred to as M0 CSPC or M1 CSPC. It isn't easy to distinguish stage M0 CSPC from a local recurrence after curative therapy. Histology and PSA are helpful in this context (see table Probability of local or systemic recurrence for patients with rising PSA after radical prostatectomy).
M1 CSPC with high tumor volume:
M1 CSPC with high tumor volume is a castration-sensitive prostate cancer with at least four bone metastases (including at least one beyond the pelvis or spine) or the presence of visceral metastases.
M1 CSPC with high risk:
M1 CSPC with high high risk is a castration-sensitive prostate cancer with at least two of the following risk factors: Gleason score ≥8, at least three bone metastases, or the presence of visceral metastases.
Oligometastatic prostate carcinoma:
Oligometastatic prostate carcinoma is a metastatic castration-sensitive prostate carcinoma with a maximum of 2–4 visible bone metastases (depending on the study) on conventional imaging.
Castration-resistant prostate carcinoma (CRPC):
Castration-resistant prostate carcinoma (CRPC) is an advanced prostate carcinoma with biochemical (rising PSA) or radiological progression despite sufficient androgen deprivation.
M0 CRPC:
M0 CRPC is the biochemical progression of prostate carcinoma under sufficient androgen deprivation and without evidence of metastases on imaging.
M0 CRPC with high risk:
M0 CRPC with a PSA doubling time of less than 10 months.
M1 CRPC:
M1 CRPC is the radiological visibleprogression of prostate carcinoma despite sufficient androgen deprivation.
Prognosis of metastatic prostate carcinoma:
Low tumor volume, no pain, no visceral metastases, metachronous metastases after previously performed local therapy, low Gleason score, a low PSA concentration, good PSA response with hormonal therapy, and a long PSA doubling time are favorable prognostic factors, see table Prognosis of metastatic prostate carcinoma.
| Time to CRPC | Median OS | |
| LT/LV | 26 months | 92 months |
| LT/HV | 15 months | 55 months |
| DN/LV | 18 months | 52 months |
| DN/HV | 12 months | 43 months |
Treatment Options
The following options exist for the treatment of advanced and metastatic prostate cancer:
- Surgical androgen deprivation therapy: bilateral subcapsular orchiectomy
- Medical androgen deprivation therapy with
- GnRH agonists (buserelin, goserelin, histrelin, leuprorelin, triptorelin)
- GnRH antagonists (abarelix, degarelix, relugolix)
- Non-steroidal androgen receptor antagonists (flutamid, bicalutamid)
- Chemotherapy with docetaxel, second-line therapy with cabazitaxel
- Androgen synthesis inhibitor (abiraterone)
- Modern androgen receptor antagonists (enzalutamide, apalutamide, daralutamide).
- Radiotherapy or surgical stabilization of metastases
- Intravenous radionuclides
- Inhibition of osteoclasts with zoledronate or denosumab
- Radiotherapy or surgical therapy of the primary tumor in oligometastatic patients.
The individual therapy options are applied sequentially or in combination depending on disease progression and response [fig. Hormonal therapy of prostate cancer]. See also pharmacokinetics and side effects of androgen deprivation therapy. Review literature: (Albers et al., 2020).
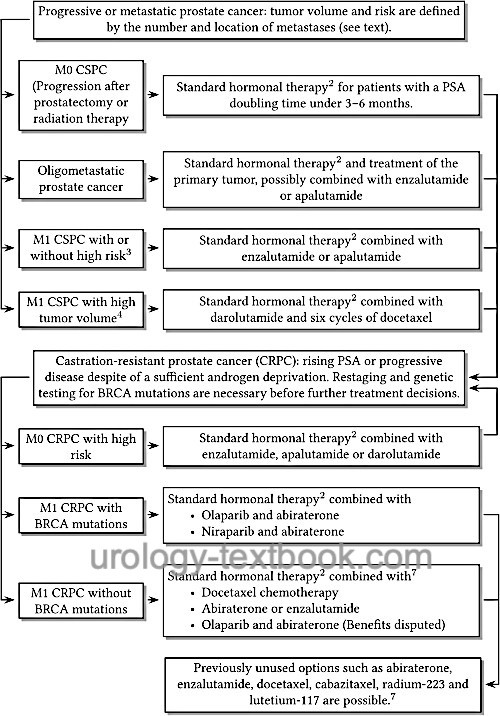 |
Treatment of Castration-Sensitive Prostate Carcinoma
Indications For Hormonal Therapy in Prostate Cancer:
The German guideline for prostate cancer recommends hormonal therapy for patients with a PSA doubling time <3 months, symptomatic local progression, proven distant metastasis, and adjuvant in the setting of radiotherapy or for patients with (extensive) lymph node metastasis after radical prostatectomy.
Standard androgen deprivation:
Most commonly, androgen deprivation is performed with the help of GnRH agonists (e.g., goserelin or leuprorelin). Since there is a temporary increase in testosterone concentration at the beginning of therapy, flutamide or bicalutamide is additionally administered p.o. for 2–4 weeks. GnRH agonists are available in the form of depot injections (every 1–6 months, depending on the preparation). Other alternatives of androgen deprivation therapy are bilateral subcapsular orchiectomy, monotherapy with non-steroidal androgen receptor antagonists (flutamide, bicalutamide), or GnRH antagonists.
Comparative trials:
Compared with orchiectomy, GnRH agonists are oncologically equivalent, and androgen receptor antagonists are only slightly inferior. The side effect profile of the androgen receptor antagonists is more favorable. GnRH antagonists lower the testosterone concentration more rapidly than GnRH antagonists but are less well tolerated and must be administered monthly.
Pharmacology and Side Effects of Hormonal Therapy:
For details on the pharmacology of hormonal therapy, the side effects of androgen ablation, and its prevention, please see the section pharmacology/hormonal therapy.
Follow-up for Patients With Hormonal Therapy
Standard imaging for detecting metastases consists of CT (abdomen and thorax) and bone scintigraphy. The additional benefit of modern imaging (PSMA-PET) still needs to be determined. Imaging should be repeated if the disease progresses to CRPC.
Laboratory controls: every 3–6 months PSA concentration. Depending on symptoms or progression, blood count, creatinine, liver enzymes, AP, and testosterone.
Prognosis:
77% of the patients will survive less than 5 years, 16% will survive 5–10 years, and 7% more than 10 years. Predictors for long-term survival are: minimal disease in imaging, low PSA concentration, low Gleason score, good general performance, no bone pain, good PSA response with hormonal therapy, and a long PSA doubling time. There are several nomograms for the survival time prediction: (Halabi et al., 2003) (Smaletz et al., 2002).
Intermittent Androgen Deprivation Therapy for Prostate Cancer
The aim of intermittent androgen deprivation therapy (IAD) is to reduce the rate of side effects by minimizing time under hormone therapy. Several studies demonstrated better libido, erection function, general physical well-being, and less hot flashes. Intermittent androgen deprivation therapy is begun until a certain PSA-Nadir is reached, which depends on the initial PSA concentration. A therapy pause follows until a specific PSA progress is reached, which again leads to another cycle of hormone therapy [fig. flow chart of intermittent androgen deprivation therapy (IAD)]. The testosterone concentration reaches normal values again, even after several cycles of hormone therapy (Pether and Goldenberg, 2004).
The oncological equivalence of intermittent hormonal therapy compared to continuous hormone therapy is debated. Several randomized studies have demonstrated oncological equivalence (Crook et al., 2012) (Mottet et al., 2012) (Salonen et al., 2012). In the largest randomized study (SWOG 9346, n = 1535), however, a lower life expectancy was observed in the group of intermittent hormonal therapy (5.1 vs. 5.8 years, 7 years survival rate 38% vs. 42%). This was even more pronounced in the patient group with low disease volume (Hussain, 2012).
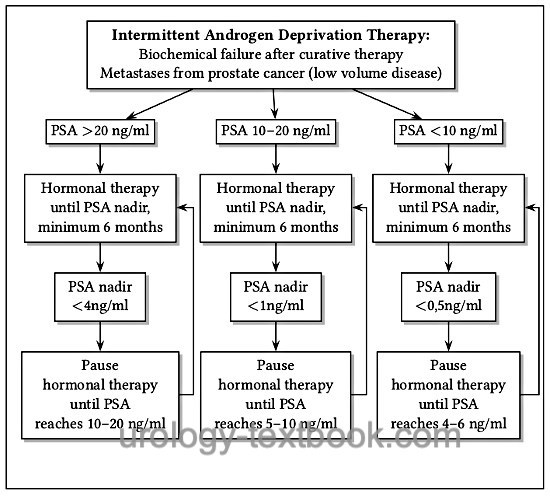 |
Local therapy for metastatic prostate cancer:
Radiotherapy of the primary tumor (in addition to systemic hormonal therapy and radiotherapy of individual metastases, if necessary) leads to a better survival rate of 7% within three years in oligometastatic patients (Burdett et al., 2019). An oncologic benefit of radical prostatectomy in oligometastatic patients is more unclear, but it is an option in selected cases (Leyh et al., 2017), especially in patients with urinary retention or LUTS.
Chemotherapy Combined With Androgen Deprivation Therapy for M1 CSPC with High Tumor Volume
Until recently, there have been no established concepts to use chemotherapy alone or in combination with hormonal therapy to treat hormone-sensitive prostate carcinoma. This has changed with the results of the STAMPEDE and CHAARTED trials (Gillessen et al., 2015). Chemotherapy should be started within the first few months after initiation of hormonal therapy.
The CHAARTED trial randomized 790 patients: one group received standard hormone therapy with GnRH antagonists or analogs; the second group received six cycles of docetaxel chemotherapy in addition to hormone therapy. In the group with high volume disease, there was a clear survival advantage of 17 months for docetaxel in addition to hormone therapy (mean survival time 49 vs. 32 months). A high volume disease was defined with at least four bone metastases (including at least one outside spine or pelvis) or the presence of visceral metastases (Sweeney et al., 2015). In the STAMPEDE study, 2962 patients were randomized: hormone therapy versus additional docetaxel (6 cycles) versus additional zoledronic acid versus additional docetaxel and zoledronic acid. The combination of hormone therapy with docetaxel extended survival by ten months (71 versus 81 months). No survival advantage could be demonstrated for zoledronic acid (James et al., 2016). In contrast to the STAMPEDE and CHAARTED trials, no benefit in overall survival could be demonstrated in the GETUG 15 trial (Gravis et al., 2013).
Triple combinations in M1 CSPC with high tumor volume:
In randomized trials, the triple combination of standard hormone therapy intensified with abiraterone or darolutamide and docetaxel chemotherapy showed benefits in terms of survival and disease progression. Triple therapy in the PEACE-1 trial (hormone therapy + abiraterone + docetaxel) resulted in better survival (HR 0.75) and prolonged progression-free survival (HR 0.5) compared with hormone therapy and docetaxel (Fizazi et al., 2022). Triple therapy in the ARASENS trial (hormone therapy + darolutamide + docetaxel) resulted in better survival (HR 0.68) and prolonged progression-free survival compared with hormone therapy and docetaxel (Smith et al., 2022a). The additional side effects from intensified hormone therapy are acceptable and probably less with darolutamide than with abiraterone. Triple therapy with darolutamide has been approved in the United States since 2022 and in Europe since 2023 and is considered the new standard for patients with a high tumor volume.
Enzalutamide combined with androgen deprivation therapy for M1 CSPC:
The ENZAMET trial (n=1125) demonstrated that androgen deprivation combined with enzalutamide improved overall survival (102 versus 143 deaths at three years) and slowed disease progression (Davis et al., 2019). The benefits were present in all risk groups. The ARCHES trial confirmed the advantage of enzalutamide (Armstrong et al., 2021); it has been approved for the therapy of hormone-sensitive prostate cancer in all risk groups.
Apalutamide combined with androgen deprivation therapy for M1 CSPC:
The TITAN trial (n=1052) demonstrated that androgen deprivation combined with apalutamide improved overall survival (HR 0.67) and slowed disease progression (HR 0.48) \parencite{Chi2019}. Apalutamide has been approved for the treatment of hormone-sensitive prostate cancer in all risk groups since 1/2020.
Abiraterone combined with androgen deprivation therapy for M1 CSPC with high risk:
The approval of abiraterone was based on the study results of STAMPEDE (James et al., 2017) and LATITUDE (Fizazi et al., 2017). Combining androgen deprivation with abiraterone 1000 mg/d and prednisolone 5 mg/d resulted in improved survival (83% vs. 76% and 66% vs. 59%, respectively) and prolonged progression-free survival and time to initiation of chemotherapy. Prerequisite is castration-sensitive prostate cancer with at least two of the following risk factors: Gleason score ≥8, at least three bone metastases, or evidence of visceral metastases. Due to the important role of abiraterone in castration-resistant prostate cancer (in combination with olaparib), its prescription should be withheld in patients with CSPC.
| Prostate cancer: TURP, HIFU | Index | Castration resistant prostate cancer |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Akduman und Crawford 2003 AKDUMAN, B. ; CRAWFORD, E. D.: The management of high risk prostate cancer.In: J Urol
169 (2003), Nr. 6, S. 1993–8
Halabi u.a. 2003 HALABI, S. ; SMALL, E. J. ;
KANTOFF, et a.:
Prognostic model for predicting survival in men with
hormone-refractory metastatic prostate cancer.
In: J Clin Oncol
21 (2003), Nr. 7, S. 1232–7
Hussain, M.
Intermittent (IAD) versus continuous
androgen deprivation (CAD) in hormone sensitive metastatic prostate cancer
(HSM1PC) patients (pts): Results of S9346 (INT-0162), an international
phase III trial.
J Clin Oncol, 2012, 30 (suppl;
abstr 4).
Loblaw u.a. 2004 LOBLAW, D. A. ; MENDELSON,
D. S. ; TALCOTT, J. A. ; VIRGO, K. S. ;
SOMERFIELD, M. R. ; BEN-JOSEF, E. ; MIDDLETON,
R. ; PORTERFIELD, H. ; SHARP, S. A. ; SMITH,
T. J. ; TAPLIN, M. E. ; VOGELZANG, N. J. ; WADE,
Jr. ; BENNETT, C. L. ; SCHER, H. I.:
American Society of Clinical Oncology recommendations for the initial
hormonal management of androgen-sensitive metastatic, recurrent, or
progressive prostate cancer.
In: J Clin Oncol
22 (2004), Nr. 14, S. 2927–41
Mottet, Nicolas; Damme, Jean Van; Loulidi, Salim;
Russel, Christoph; Leitenberger, Armin; Wolff, Johannes M & the TAP22
Investigators Group
Intermittent hormonal therapy in the treatment of
metastatic prostate cancer: a randomized trial.
BJU Int, 2012.
M. Pether and S. L. Goldenberg.
Intermittent androgen suppression.
BJU Int, 93 (3): 258–261, Feb 2004.
Pound u.a. 1999 POUND, C. R. ; PARTIN, A. W. ;
EISENBERGER, M. A. ; CHAN, D. W. ; PEARSON,
J. D. ; WALSH, P. C.:
Natural history of progression after PSA elevation following radical
prostatectomy.
In: Jama
281 (1999), Nr. 17, S. 1591–7
N. Mottet (Chair), J. Bellmunt, E. Briers (Patient Representative), R.C.N. van den Bergh (Guidelines Associate),
M. Bolla, N.J. van Casteren (Guidelines Associate), P. Cornford, S. Culine, S. Joniau, T. Lam, M.D. Mason, V. Matveev, H. van der Poel, T.H. van der Kwast, O. Rouvière, T. Wiegel
Guidelines on Prostate Cancer of the European Association of Urology (EAU), https://uroweb.org/guidelines/prostate-cancer/.
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe,
AWMF):
Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms, Langversion 3.1, 2014 AWMF Registernummer: 034/022OL, https://www.awmf.org//leitlinien/detail/ll/043-022OL.html (Zugriff am: 07.02.2016)
Wein, A. J.; Kavoussi, L. R.; Partin, A. P. & Peters, C. A.
Campbell-Walsh Urology
. Elsevier, 2015. ISBN 978-1455775675.
Wein, A. J.; Kavoussi, L. R.; Partin, A. P. & Peters, C. A.
Campbell-Walsh Urology
. Elsevier, 2015. ISBN 978-1455775675.
Salonen, A. J.; Taari, K.; Ala-Opas, M.; Viitanen, J.;
Lundstedt, S.; Tammela, T. L. J. & Group, F.
The FinnProstate Study
VII: intermittent versus continuous androgen deprivation in patients with
advanced prostate cancer.
J Urol, 2012, 187,
2074-2081.
Smaletz u.a. 2002 SMALETZ, O. ; SCHER, H. I. ;
SMALL, E. J. ; VERBEL, D. A. ; MCMILLAN, A. ;
REGAN, K. ; KELLY, W. K. ; KATTAN, M. W.:
Nomogram for overall survival of patients with progressive metastatic
prostate cancer after castration.
In: J Clin Oncol
20 (2002), Nr. 19, S. 3972–82
 Deutsche Version: Diagnose und Therapie des Prostatakarzinoms
Deutsche Version: Diagnose und Therapie des Prostatakarzinoms