You are here: Urology Textbook > Prostate > Prostate cancer > Staging
Prostate Cancer: Diagnosis and Staging
- Prostate cancer: Epidemiology and etiology
- Prostate cancer: Pathology
- Prostate cancer: Signs and symptoms
- Prostate cancer: Screening
- Prostate cancer: Staging
- Prostate cancer: Treatment options
- Prostate cancer: Active surveillance
- Prostate cancer: Prostatectomy
- Prostate cancer: Radiation therapy
- Prostate cancer: Brachytherapy
- Prostate cancer: TURP and experimental treatment options
- Prostate cancer: Hormonal therapy of advanced prostate cancer
- Prostate cancer: Treatment of castration-resistant prostate cancer
Guidelines and review literature: (EAU Guidelines Prostate Cancer) (S3-Leitlinie Prostatakarzinom) (Walsh-Campbell Urology).
Of crucial importance for any therapeutic decision is both the assessment of the tumor stage and the evaluation of the biological aggressiveness of the prostate cancer. Staging includes digital-rectal examination (DRE), PSA concentration, results of the prostate needle biopsy, laboratory tests, imaging, and pelvic lymphadenectomy.
Clinical Staging of Prostate Cancer
Clinical staging is done with digital-rectal examination, PSA concentration, and Gleason score of the prostate biopsy. Each parameter has a statistical correlation with the pathologic tumor stage. The prediction accuracy of a single parameter is too low in individual patients. The prediction of the clinical risk (prostate cancer mortality) is accurate with the combined consideration [see table risk stratification of prostate cancer].
| Risk | Criteria | 10-year mortality after EBRT (*) | 10-year risk of PSA Prgression after RPE | 10-year mortality after RPE (*) |
| Low | PSA <10 ng/ml and Gleason score ≤6 | 2% | 17% | 1% |
| Intermediate | PSA 10–20 ng/ml or Gleason score 7 | 8% | 54% | 4% |
| High | PSA >20 ng/ml or Gleason score >7 | 24% | 71% | 11% |
Another important risk factor is PSA velocity in the year before treatment; unfavorable is a rise of more than 2 ng/ml (D'Amico et al., 2006). With additional consideration of the tumor burden in the prostate biopsy, the prediction accuracy will be even better. With additional consideration of the tumor burden in the prostate biopsy, the prediction accuracy will be even better (CAPRA score), see table CAPRA-Score. CAPRA stands for Cancer of the Prostate Risk Assessment and was developed by the University of California, San Francisco based on data from the CaPSURE study (Cooperberg et al., 2009). See table pathologic outcomes and prognosis after radical prostatectomy as a function of the CAPRA score.
| Risk factor | Criteria | Points |
| Age | < 50 years | 0 |
| > 50 years | 1 | |
| PSA | < 6 ng/ml | 0 |
| 6,1–10 ng/ml | 1 | |
| 10,1–20 ng/ml | 2 | |
| 20,1–30 ng/ml | 3 | |
| > 30 ng/ml | 4 | |
| > 30 ng/ml | 4 | |
| Biopsy Gleason score | No pattern 4 or 5 | 0 |
| Secondary pattern 4 oder 5 | 1 | |
| Primary pattern 4 oder 5 | 3 | |
| Clinical stage | T1–2 | 0 |
| ≥ T3a | 1 | |
| Positive cores in biopsy | < 34% | 0 |
| > 34% | 1 |
| CAPRA score | 0–1 | 2 | 3 | 4 | 5 | 6 | >7 |
| Positive surgical margin | 8–24 | 15–26 | 16–31 | 18–42 | 26–44 | 44–53 | 42–58 |
| Extracapsular extension | 6–14 | 16–19 | 23–26 | 26–40 | 44–55 | 51–56 | 68–86 |
| Seminal vesicle infiltration | 1–2 | 3–4 | 4–6 | 9–11 | 13–21 | 22 | 27–47 |
| Lymph node metastasis | 0–1 | 1–3 | 2 | 1–4 | 3–11 | 4–11 | 3–34 |
| 3 years progression free | 92–95 | 84–95 | 76–87 | 73–82 | 67–70 | 46–72 | 35–50 |
| 5 years progression free | 86–91 | 75–91 | 65–83 | 60–74 | 52–61 | 29–62 | 20–30 |
The Partin tables combine local tumor stage, PSA level, and Gleason score to predict pathology results after radical prostatectomy. The tables are based on the pathological results at the James Buchanan Brady Urological Institute. Some authors think that the Partin tables underestimate the risk of lymph node metastasis. The reason may be the limited dissection field used in this clinical series.
The Kattan nomograms of the Memorial Sloan Kettering Cancer Center also use the combination of local tumor stage, PSA concentration and Gleason sum. As a result of the nomogram, the investigator receives the 10-year progression-free survival rate after radical prostatectomy and the 15-year cancer-specific survival rate.
Bone Scintigraphy
Bone scan is used to diagnose bone metastases of prostate cancer. It has a relatively high sensitivity with poor specificity [figure prostate cancer bone metastasis]. Suspect lesions need further diagnostic workup with X-rays, CT, MRI, or bone biopsy.
For PSA concentration below 10 ng/ml, the rate of bone metastases in prostate cancer is very low (1.3%) at a constant rate of false-positive findings. In patients with an organ-confined tumor, PSA less than 10 ng/ml, a Gleason score below, 8 and missing bone pain, bone scintigraphy can be safely omitted before curative treatment.
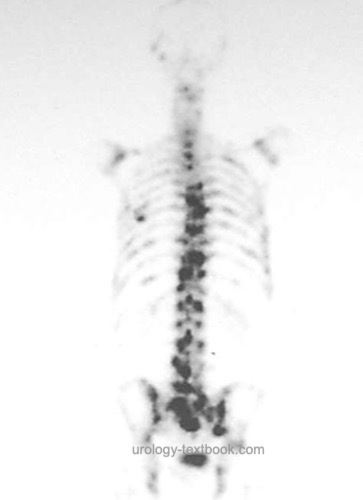 |
Computed Tomography
A CT scan is able to identify enlarged lymph nodes, hydronephrosis, bone metastases and infiltration of neighboring organs of the primary tumor. In patients with a PSA below 20 ng/ml and a Gleason score below 8, the pathologies mentioned above are rare. The significance of enlarged lymph nodes is limited (not specific and may be false-positive). A CT scan is indicated in patients with high-risk prostate carcinoma, either in combination with bone scintigraphy or PET (see next section).
Positron Emission Tomography
PET (in combination with a CT or MRI) yields high diagnostic accuracy and is superior to the combination of bone scintigraphy and CT (Hofman u.a., 2020). 68Ga-PSMA is used as a tracer. Indications for PSMA-PET-CT include high-risk prostate cancer, biochemical recurrence after curative therapy, or progression with systemic treatment for metastatic prostate cancer.
Multiparametric MRI of the Prostate
MRI of the prostate is indicated for imaging of locally advanced prostate cancer if imaging results would change the treatment decision.
Pelvic Lymphadenectomy
Pelvic lymphadenectomy is necessary for the exact diagnosis of nodal metastasis. Any imaging technique can only detect lymph node enlargement and is, therefore, neither sensitive nor specific. Surgical (laparoscopic) nodal staging as an isolated intervention is seldom indicated in patients with a higher risk of lymph node metastasis, particularly before perineal prostatectomy or brachytherapy. See section laparoscopic pelvic lymphadenectomy for details of the procedure.
The dissection field of pelvic lymphadenectomy is debated (limited versus extended). The boundaries for a limited dissection are:
- Lateral boundary: external iliac artery
- Kaudal boundary: superior ramus of the pubis
- Cranial boundary: bifurcation of the common iliac artery
- Medial boundary: bladder, branches of the internal iliac artery
- Dorsal boundary: obturator fossa
In low-risk tumors (Gleason score <7 and PSA < 10 ng/ml), the probability of lymph node metastasis is very low (< 5%). Pelvic lymphadenectomy is unnecessary before curative treatment. In low-risk patients, the need for surgical lymph node staging, even during retropubic prostatectomy, is controversial and debated. Some authors believe that the incidence of lymph node metastases is underestimated (e.g., in the Partin tables, see above). They demand to perform extended pelvic lymphadenectomy if radical prostatectomy is necessary.
As an alternative for extended lymphadenectomy in low-risk patients, some centers perform sentinel lymphadenectomy. After injection of a radioactive tracer in the prostate, sentinel lymph nodes are intraoperatively detected with a gamma probe and removed. Sentinel lymphadenectomy limits the dissection field, and sensitivity is higher than in standard lymphadenectomy (Wawroschek et al., 2003). Controlled trials for sentinel lymphadenectomy are not available; the procedure is time-consuming and expensive.
Experimental Diagnostic Procedures
New Tumor Markers for Screening
PCA3 (prostate cancer antigen 3)
PCA3 is a non-protein-coding RNA, which is highly overexpressed in prostate cancer cells and can be detected in the urine after prostate massage (DRE). Initial studies indicate a better sensitivity and specificity for PCA3 in men with elevated PSA. In addition, PCA3 may play a role in future treatment algorithms, since tumor volume can be predicted (Auprich et al., 2011). In Germany, the PCA3 tests cost around 300 euros.
Analysis of Gene Expression
Prolaris is a diagnostic test from Myriad Genetics, which analyzes the RNA expression of cell cycle genes in prostate biopsy tissue. Thirty proliferation genes are examined, and 15 housekeeping genes serve as controls (Cuzick et al., 2012). The test claims to increase the safety for patients with low-risk cancer choosing active surveillance, but controlled studies are lacking. The test is costly (around 3000 dollars).
Other Methods:
Detection of micrometastases in bone marrow, RT-PCR for detecting prostate cancer cells in the blood or lymph nodes.
New Techniques for Imaging
New methods and tracers improve imaging with PET, MRI, or ultrasound.
Micro-ultrasound:
Targeted prostate biopsy with a high-resolution TRUS probe (ExactVu with 29 MHz) was able to achieve better results than conventional systematic biopsy in several studies (Dariane et al., 2022).
Elastography:
The elasticity of the prostate tissue can be displayed in color with the help of a special ultrasonic probe and pressure on the prostate. Less elastic regions are suspicious for cancer. Elastography is better in prostate cancer detection than normal gray-scale imaging. The sensitivity and specificity of elastography is 60–70%, but this not sufficient to omit prostate biopsy in patients with elevated PSA if imaging appears normal (Brock et al., 2012). In addition, elastography helps target prostate biopsy.
Analysis of the Ultrasonography Raw Data:
HistoScanning is a database-assisted analysis of the raw data from the ultrasound probes and is supposed to be better than conventional gray-scale imaging to detect prostate cancer. HistoScanning is already available for a fee (per application 200–500 Euros). Independent trials could not confirm initial promising results (Schiffmann et al., 2014) (Javed et al., 2014).
MRI imaging:
MRI with magnetic nanoparticles or diffusion-weighted imaging (DWI) increases the detection accuracy of lymph node metastases (Harisinghani et al., 2003).
| Prostate cancer screening | Index | Prostate cancer treatment options |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
Afshar-Oromieh, A.; Haberkorn, U.; Schlemmer, H. P.;
Fenchel, M.; Eder, M.; Eisenhut, M.; Hadaschik, B. A.; Kopp-Schneider, A.
& Röthke, M.
Comparison of PET/CT and PET/MRI hybrid systems
using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate
cancer: initial experience.
Eur J Nucl Med Mol Imaging, 2014,
41, 887-897
Auprich, M.; Bjartell, A.; Chun, F. K.; de la Taille,
A.; Freedland, S. J.; Haese, A.; Schalken, J.; Stenzl, A.; Tombal, B. &
van der Poel, H.
Contemporary role of prostate cancer antigen 3 in the
management of prostate cancer.
Eur Urol, 2011, 60,
1045-1054.
Brock, M.; von Bodman, C.; Palisaar, R. J.; Löppenberg,
B.; Sommerer, F.; Deix, T.; Noldus, J. & Eggert, T.
The Impact of
Real-Time Elastography Guiding a Systematic Prostate Biopsy to Improve
Cancer Detection Rate: A Prospective Study of 353 Patients.
J Urol, 2012.
D'Amico, A. V.; Moul, J.; Carroll, P. R.; Sun, L.;
Lubeck, D. & Chen, M.
Cancer-specific mortality after surgery or
radiation for patients with clinically localized prostate cancer managed
during the prostate-specific antigen era.
J Clin Oncol, 2003,
21, 2163-2172.
D'Amico, A. V.; Hui-Chen, M.; Renshaw, A. A.; Sussman,
B.; Roehl, K. A. & Catalona, W. J.
Identifying men diagnosed with
clinically localized prostate cancer who are at high risk for death from
prostate cancer.
J Urol, 2006, 176, S11-S15.
Guidelines on Prostate Cancer of the European Association of Urology (EAU), https://uroweb.org/guidelines/prostate-cancer/.
Harisinghani u.a. 2003 HARISINGHANI, M. G. ;
BARENTSZ, J. ; HAHN, P. F. ; DESERNO, W. M. ;
TABATABAEI, S. ; KAA, C. H. van de ; ROSETTE, J.
de la ; WEISSLEDER, R.:
Noninvasive detection of clinically occult lymph-node metastases in
prostate cancer.
In: N Engl J Med
348 (2003), Nr. 25, S. 2491–9
Javed, S.; Chadwick, E.; Edwards, A. A.; Beveridge, S.;
Laing, R.; Bott, S.; Eden, C. & Langley, S.
Does prostate
HistoScanning™ play a role in detecting prostate cancer in routine
clinical practice? Results from three independent studies.
BJU Int, 2014,
114, 541-548
Kattan u.a. 1998 KATTAN, M. W. ; EASTHAM,
J. A. ; STAPLETON, A. M. ; WHEELER, T. M. ;
SCARDINO, P. T.:
A preoperative nomogram for disease recurrence following radical
prostatectomy for prostate cancer.
In: J Natl Cancer Inst
90 (1998), Nr. 10, S. 766–71
Kattan u.a. 2001 KATTAN, M. W. ; POTTERS, L. ;
BLASKO, J. C. ; BEYER, D. C. ; FEARN, P. ;
CAVANAGH, W. ; LEIBEL, S. ; SCARDINO, P. T.:
Pretreatment nomogram for predicting freedom from recurrence after
permanent prostate brachytherapy in prostate cancer.
In: Urology
58 (2001), Nr. 3, S. 393–9
Kattan u.a. 2003 KATTAN, M. W. ; ZELEFSKY,
M. J. ; KUPELIAN, P. A. ; CHO, D. ; SCARDINO,
P. T. ; FUKS, Z. ; LEIBEL, S. A.:
Pretreatment nomogram that predicts 5-year probability of metastasis
following three-dimensional conformal radiation therapy for localized
prostate cancer.
In: J Clin Oncol
21 (2003), Nr. 24, S. 4568–71
Perdonà, S.; Cavadas, V.; Lorenzo, G. D.; Damiano, R.;
Chiappetta, G.; Prete, P. D.; Franco, R.; Azzarito, G.; Scala, S.; Arra,
C.; Sio, M. D. & Autorino, R.
Prostate Cancer Detection in the
"Grey Area" of Prostate-Specific Antigen Below 10 ng/ml: Head-to-Head
Comparison of the Updated PCPT Calculator and Chun's Nomogram, Two Risk
Estimators Incorporating Prostate Cancer Antigen 3.
Eur Urol, 2010
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms, https://www.leitlinienprogramm-onkologie.de/leitlinien/prostatakarzinom
Wein, A. J.; Kavoussi, L. R.; Partin, A. P. & Peters, C. A.
Campbell-Walsh Urology
. Elsevier, 2015. ISBN 978-1455775675.
Sanz u.a. 2004 SANZ, G. ; RIOJA, J. ;
ZUDAIRE, J. J. ; BERIAN, J. M. ; RICHTER, J. A.:
PET and prostate cancer.
In: World J Urol
22 (2004), Nr. 5, S. 351–2
Schiffmann, J.; Tennstedt, P.; Fischer, J.; Tian, Z.;
Beyer, B.; Boehm, K.; Sun, M.; Gandaglia, G.; Michl, U.; Graefen, M. &
Salomon, G.
Does HistoScanning™ predict positive results in prostate
biopsy? A retrospective analysis of 1,188 sextants of the prostate.
World
J Urol, 2014, 32, 925-930.
Smaletz u.a. 2002 SMALETZ, O. ; SCHER, H. I. ;
SMALL, E. J. ; VERBEL, D. A. ; MCMILLAN, A. ;
REGAN, K. ; KELLY, W. K. ; KATTAN, M. W.:
Nomogram for overall survival of patients with progressive metastatic
prostate cancer after castration.
In: J Clin Oncol
20 (2002), Nr. 19, S. 3972–82
Wawroschek u.a. 2003 WAWROSCHEK, Friedhelm ;
WAGNER, Theodor ; HAMM, Michael ; WECKERMANN,
Dorothea ; VOGT, Harry ; MäRKL, Bruno ; GORDIJN,
Ronald ; HARZMANN, Rolf:
The influence of serial sections, immunohistochemistry, and extension
of pelvic lymph node dissection on the lymph node status in clinically
localized prostate cancer.
In: Eur Urol
43 (2003), Feb, Nr. 2, S. 132–6; discussion 137
 Deutsche Version: Prostatakarzinom
Deutsche Version: Prostatakarzinom
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.