You are here: Urology Textbook > Kidneys > Renal cell carcinoma > Treatment of advanced renal cell carcinoma
Treatment of Metastatic Renal Cell Carcinoma
- Renal cell carcinoma: Definition and Epidemiology
- Renal cell carcinoma: Pathology
- Renal cell carcinoma: Diagnostic workup
- Renal cell carcinoma: Surgical Treatment
- Renal cell carcinoma: Targeted therapy of Metastases
Prognosis of Metastatic Renal Cell Carcinoma
Motzer from the MSKCC developed accepted criteria for the prognosis of metastatic renal cell carcinoma, which are used for the stratification of patients within trials of immunotherapy (Motzer et al., 2002); see table Motzer criteria. The IMDC score is an update with patient data from modern studies with kinase inhibitors and checkpoint inhibitors (Heng et al., 2013); see table IMDC score for survival time of the risk groups.
.
| Adverse risk factor | Limit |
| Reduced Karnofsky Index | <80% |
| Elevated LDH | >1.5 times the upper limit of normal |
| Low hemoglobin | below the lower limit of normal |
| Elevated corrected calcium | > 10 mg/dl or 2,4 mmol/l |
| Time from nephrectomy to metastases formation | below one year |
.
Surgical Therapy of Primary Tumor And Metastases
If complete resection of all metastases and the primary tumor is possible, surgical therapy should be done without neoadjuvant therapy. Depending on the location of the metastases (lungs, liver, bones), appropriate surgical specialists are needed. Despite the complete resection of all visible lesions, there is a high risk of recurrence. Still, surgical therapy can delay drug therapy, improve the patient's prognosis, and alleviate local complaints.
The prognosis after removal of a solitary metastasis is less favorable in patients for whom the metastasis already existed at the initial diagnosis. If the metastasis is discovered during the follow-up, the time from initial diagnosis to metastasis is crucial for the prognosis.
Cytoreductive nephrectomy in patients with metastatic renal cell carcinoma:
Cytoreductive nephrectomy is the primary tumor resection before drug therapy for metastases. In addition to palliation of local complaints, nephrectomy before classic immunotherapy leads to an improved survival (Mickisch u.a., 2001a). Laparoscopic cytoreductive nephrectomy offers advantages due to the shorter convalescence time.
Two randomized studies (CARMENA and SURTIME) were carried out for cytoreductive nephrectomy prior to therapy with signal transduction inhibitors. Neither study demonstrated any benefits for cytoreductive nephrectomy before sunitinib (Mejean et al., 2018) (Bex et al., 2019), but only patients with medium or high risk were included.
The current EAU guideline recommends cytoreductive nephrectomy only for patients with a small metastasis volume and a large primary tumor. If the patient responds well to targeted therapy, delayed cytoreductive nephrectomy is an option. There is no indication for cytoreductive nephrectomy in patients with high risk, small primary tumor and high metastatic burden, poor performance status, Bellini duct carcinoma, or sarcomatoid tumors (if the histology is known before surgery).
Checkpoint inhibitors
 |
The activation of immune checkpoint receptors leads to the inhibition and weakening of the cellular immune response. They have a physiological function in preventing autoimmune diseases. Various receptors have been identified: PD-1 receptor (PD for programmed cell death) or CTLA-4 (CTLA for cytotoxic T lymphocyte antigen) on T lymphocytes with corresponding ligands such as PD-L1. Tumors secrete ligands to immune checkpoints to induce immune tolerance. See section pharmacology and side effects of checkpoint inhibitors (CPI).
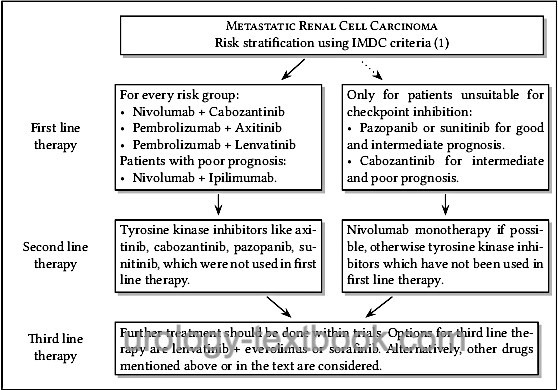
The superiority of the combined therapy (tyrosine kinase inhibitor with immune checkpoint inhibitor) over the monotherapy was demonstrated in several studies. Therapy leads up to 10% to a complete response and often to a significant partial response, partly the treatment effect is delayed. Side effects are substantial (15% grade 3–4, 1.5% treatment-related deaths). A switch to the next option is necessary only if there is clear progress or intolerable side effects (despite adjuvant therapy and dose reduction), see fig. sequential therapy of metastatic renal cell carcinoma.
Avelumab combined with axitinib:
The combination improved (compared to sunitinib) progression-free survival (14 vs. 7 months) and response rate (CR 4% vs. 2% and partial response 49% vs. 25%); data for survival have not yet been published (Motzer et al., 2019). The benefit was independent of the IMDC risk classification.
Nivolumab monotherapy:
Nivolumab monotherapy as a second-line therapy after TKI failure (compared to everolimus) improved progression-free survival, response rate (CR + PR: 25% vs. 5%), overall survival (25 vs. 20 months), and showed fewer side effects (Motzer et al., 2015).
Nivolumab combined with cabozantinib:
The CheckMate-9ER study showed improved progression-free survival (17 vs. 8 months, HR 0.51), overall response (56% vs. 27%), complete response (8% vs. 5%) and overall survival (HR 0.60) compared to sunitinib, the benefit was detectable in all risk groups (Choueiri et al., 2021a).
Nivolumab combined with ipilimumab:
The CheckMate-214 study showed significant advantages for nivolumab combined with ipilimumab in progression-free survival and overall survival (56 vs. 38 months after five years follow-up) compared to sunitinib in first-line therapy of patients with intermediate and poor prognosis. The combination has also a good response in patients with sarcomatoid renal cell carcinoma (Albiges et al., 2020).
Pembrolizumab combined with axitinib:
Improved progression-free survival, response rate (CR 6% vs. 2%, PR 53% vs. 34%), and overall survival (HR 0.59, 1-year survival rate 90% vs. 79%) (Rini et al., 2019); this was independent of the IMDC risk classification or the PD-L1 expression.
Pembrolizumab combined with lenvatinib:
Improved progression-free survival compared to sunitinib (24 vs. 9 months) and overall survival (HR 0,66) (Motzer et al., 2021).
Targeted Therapy of Renal Cell Carcinoma With Inhibitors of Signal Transduction
Treatment with inhibitors of signal transduction (smart drugs or targeted therapy) replaced immunotherapy with interferon or interleukin. Inhibitors of signal transduction were standard of treatment before the introduction of checkpoint inhibitors. They are currently administered in combination with checkpoint inhibitors (see above), or as monotherapy when CPI are contraindicated. Inhibitors of signal transduction generate stable disease; complete remission is rare. Favorable prognostic signs are metastases that stabilize or decrease in size and show signs of decreased perfusion in computed tomography.
Targeted molecular agents show a favorable side effect profile in comparison to immunotherapy. Once started, targeted therapy must be continued as long as possible (until progression or intolerable side effects). Sequential therapy of "smart drugs" is followed by a change to the next targeted molecular agents; see flowchart sequential therapy of metastatic renal cell carcinoma.
Axitinib:
Axitinib is a potent inhibitor of VEGF receptor tyrosine kinase. Axitinib has been approved since 2012 in the U.S. for second-line treatment after failure with cytokines or first-line treatment with targeted molecules. Axitinib increased progression-free survival (6.7 vs. 4.7 months compared to sorafenib) (Rini et al., 2011). Axitinib has gained new importance in first-line therapy as a combination treatment with pembrolizumab or avelumab, see above. For dosage, pharmacology, and side effects, see chapter pharmacology section Axitinib.
Bevacizumab:
Bevacizumab is a monoclonal antibody against VEGF and has been approved for first-line treatment of metastatic renal cell carcinoma. In combination with interferon, bevacizumab shows an increase in progression-free survival of 10.2 vs. 5.4 months in comparison to interferon therapy alone (Escudier et al., 2007) (Motzer and Bukowski, 2006).
Cabozantinib:
Cabozantinib is an oral inhibitor of tyrosine kinases such as MET, VEGF, and AXL. Cabozantinib has been approved for second and third-line therapy of metastatic renal cell carcinoma. Compared to everolimus, progression-free survival (7.4 vs. 3.8 months) and overall survival (21 vs. 19 months) were prolonged (Choueiri u.a., 2015). Cabozantinib also showed a higher progression-free survival rate (9 vs. 5 months) in medium and high-risk patients in first-line therapy compared to sunitinib (Choueiri u.a., 2018), so that approval for first-line therapy in medium and low-risk patients was also granted.
Inhibitors of mTOR:
Everolimus and Temsirolimus are inhibitors of mTOR (mammalian target of rapamycin), a central molecule in the intracellular signal transduction of cell growth, angiogenesis, energy metabolism, and apoptosis (Faivre et al., 2006). Everolimus has been approved after failure of therapy with inhibitors of signal transduction (Motzer et al., 2008), there was a slight improvement (5 vs. 2 months) in progression-free survival compared to placebo. Current guidelines mention mTOR inhibitors as an option in third line therapy.
Pazopanib:
Pazopanib is a tyrosine kinase inhibitor (VEGF receptor, PDGF receptor tyrosine kinase, and c-kit) with approval for first-line treatment and second-line therapy after interferon therapy of metastasized renal cell carcinoma (Hutson et al., 2010). Progression-free survival improved significantly (9.2 vs. 4.2 months); the difference was even more pronounced in the subgroup without prior therapy (11.1 vs. 2.8 months). No significant differences could be demonstrated for overall survival.
Sorafenib:
Sorafenib is an oral multi-kinase inhibitor which engages in the intracellular signal transduction of cell growth and angiogenesis. Inhibited kinases include RAF kinase, VEGF receptor kinases, PDGF receptor kinase, KIT, and FLT-3 (Escudier et al., 2007a). Sorafenib is a treatment option after failure of first and second-line therapy. The approval study showed improved progression-free and overall survival after failure of cytokine therapy (Escudier et al., 2009). The direct comparison with sunitinib in first and second-line therapy (SWITCH1) showed no significant differences in the sequence sorafenib-sunitinib vs. Sunitinib-Sorafenib (Eichelberg et al., 2015).
Sunitinib:
Sunitinib is an oral multi-kinase inhibitor, especially of tyrosine kinases like VEGF and PDGF receptor kinases. Randomized studies with sunitinib showed an improved progression-free survival of 11 vs. 5 months compared to immunotherapy with interferon (Motzer et al., 2007). Overall survival was not significantly longer: 26 vs. 22 months. Sunitinib has been approved for first and second-line therapy after interferon therapy of metastasized renal cell carcinoma.
Tivozanib:
Tivozanib is a tyrosine kinase inhibitor of VEGF receptor kinase. Tivozanib was tested against sorafenib in a randomized study, it showed an improvement in progression-free survival (12 vs. 9 months) with better tolerability (Motzer et al., 2013). Overall survival was not improved. Tivozanib is a treatment option for first-line therapy in patients with low or intermediate risk.
Belzutifan: Belzutifan is a well-tolerated inhibitor of hypoxia-inducible factor-2α (HIF-2α). Belzutifan is approved in the USA for treating VHL-associated tumors (Jonasch et al., 2021). Belzutifan showed an improved response in third-line therapy compared with everolism: progression-free survival 34% vs. 18%, differences in overall survival were insignificant. Belzutifan has not yet been approved in Europe (as of 10/24).Immunotherapy With Interferon or Interleukin::
The treatment with interferon-α in combination with interleukin-2 and 5-FU achieves partial remission in selected patients (up to 40%). However, some randomized trials showed far less remission and missing effect on survival (Negrier et al., 2007). Immunotherapy causes significant toxicity and costs. Toxicity may be reduced with interferon-α monotherapy, but the response rate is even lower (Mancuso and Sternberg, 2005). With the emergence of targeted therapy, immunotherapy (without bevacizumab) is hardly used anymore.
Chemotherapy of metastatic renal cell carcinoma:
The following chemotherapy regimens have been tested with only moderate response: gemcitabine and 5-FU, gemcitabine in combination with immunotherapy. No chemotherapeutic regimen is accepted as a standard of care.
Palliative Treatment Options in Metastatic Renal Cell Carcinoma:
Painful bone metastases:
Pain management is standard care. Additional therapeutic options are irradiation, bisphosphonates (zoledronate), denosumab, or surgical stabilization.
Hypercalcemia:
Hypercalcemia is treated with corticosteroids in higher-dose, forced diuresis, saline infusion, and bisphosphonates.
Local pain or bleeding tumor:
Palliative nephrectomy or embolization.
Brain metastases:
Corticosteroids, irradiation (e.g., Gamma knife).
Prognosis of renal cell carcinoma
Natural history of localized renal cell carcinoma:
Renal tumors show, on average, a growth of 3–5 mm per year. Many small tumors do not grow. Metastases from renal tumors smaller than 3–cm are rare.
Clinical stage and prognosis:
See table Robson stage and survival for prognosis and clinical stage. More precise results for the survival probability are possible by considering several risk factors, see stage, size, grade, and necrosis (SSIGN) Score [(Assessing SSIGN Score and Survival and SSIGN Score)].
Venous invasion:
Almost 40% of patients with venous invasion are not suffering from metastases (pN0 and M0) and may be cured by surgery. Patients with pN0 M0 have a 5-year survival rate of 70%. 26% have lymph node metastases, 54% have distant metastases.
Grading and survival:
Five-year survival rates depending on grading: G1 (89%), G2 (65%), G3 (46%).
Prognosis of renal cell carcinoma with lymph node metastasis:
Five-year survival rate 5–30%.
Prognosis of renal cell carcinoma with systemic metastasis:
Metastatic renal cell cancer is a very aggressive disease. Patients with metastases at initial diagnosis have a poor prognosis and usually die within the first year. The interval from the nephrectomy to the onset of metastases is important for prognosis, as it provides information on the rate of progression of the systemic disease. Patients with pulmonary metastases have the best prognosis. In addition to metastases in other locations, the following factors indicate a poor prognosis: low Karnofsky index, tumor anemia, abnormal high corrected serum calcium, elevated LDH, elevated AP, elevated platelets, and elevated neutrophils. See tab. IMDC Score for mean survival time under treatment with TKI and CPI.
Prognosis of renal cell carcinoma with brain metastases:
Median survival of 7 months; prognostic factors are Karnofsky index and number of metastases.
| RCC surgical therapy | Index | Wilms-Tumor |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
L. Albiges, N. M. Tannir, M. Burotto, D. McDermott, E. R. Plimack, and R. J. Motzer, “Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial.,” ESMO open, vol. 5, no. 6, p. e001079, 2020.
Choueiri, T. K.; Escudier, B.; Powles, T.; Mainwaring,
P. N.; Rini, B. I.; Donskov, F.; Hammers, H.; Hutson, T. E.; Lee, J.-L.;
Peltola, K.; Roth, B. J.; Bjarnason, G. A.; Géczi, L.; Keam, B.; Maroto,
P.; Heng, D. Y. C.; Schmidinger, M.; Kantoff, P. W.; Borgman-Hagey, A.;
Hessel, C.; Scheffold, C.; Schwab, G. M.; Tannir, N. M.; Motzer, R. J. & ,
M. E. T. E. O. R. I.
Cabozantinib versus Everolimus in Advanced
Renal-Cell Carcinoma.
N Engl J Med, 2015, 373,
1814-1823.
DGU; DKG; DKG & Leitlinienprogramm Onkologie Diagnostik, Therapie und Nachsorge des Nierenzellkarzinoms, Langversion 4.02023 https://www.leitlinienprogramm-onkologie.de/leitlinien/nierenzellkarzinom/.
Escudier u.a. 2007 ESCUDIER, Bernard ; EISEN,
Tim ; STADLER, Walter M. ; SZCZYLIK, Cezary ;
OUDARD, Stéphane ; SIEBELS, Michael ; NEGRIER,
Sylvie ; CHEVREAU, Christine ; SOLSKA, Ewa ;
DESAI, Apurva A. ; ROLLAND, Frédéric ; DEMKOW,
Tomasz ; HUTSON, Thomas E. ; GORE, Martin ;
FREEMAN, Scott ; SCHWARTZ, Brian ; SHAN, Minghua ;
SIMANTOV, Ronit ; BUKOWSKI, Ronald M. ; GROUP, T.
A. R. G. E. T. S.:
Sorafenib in advanced clear-cell renal-cell carcinoma.
In: N Engl J Med
356 (2007), Jan, Nr. 2, S. 125–134
B. Escudier, T. K. Choueiri, S. Oudard, C. Szczylik, S. Negrier, A. Ravaud,
C. Chevreau, P. Venner, P. Champagne, D. Croteau, E. Dupont, C. Hariton, and
R. M. Bukowski.
Prognostic factors of metastatic renal cell carcinoma after failure
of immunotherapy: new paradigm from a large phase iii trial with shark
cartilage extract ae 941.
J Urol, 178 (5): 1901–1905, Nov 2007.
doi: rm10.1016/j.juro.2007.07.035.
URL https://dx.doi.org/10.1016/j.juro.2007.07.035.
B. Escudier, A. Pluzanska, P. Koralewski, A. Ravaud, S. Bracarda, C. Szczylik,
C. Chevreau, M. Filipek, B. Melichar, E. Bajetta, V. Gorbunova, J. O. Bay,
I. Bodrogi, A. Jagiello-Gruszfeld, und N. Moore.
Bevacizumab plus interferon alfa-2a for treatment of metastatic renal
cell carcinoma: a randomised, double-blind phase iii trial.
Lancet, 370 (9605): 2103–2111, Dec 2007.
S. Faivre, G. Kroemer, and E. Raymond.
Current development of mtor inhibitors as anticancer agents.
Nat Rev Drug Discov, 5 (8): 671–688, Aug
2006.
doi: rm10.1038/nrd2062.
URL https://dx.doi.org/10.1038/nrd2062.
Frank u.a. 2003 FRANK, I. ; BLUTE, M. L. ;
CHEVILLE, J. C. ; LOHSE, C. M. ; WEAVER, A. L. ;
LEIBOVICH, B. C. ; ZINCKE, H.:
A multifactorial postoperative surveillance model for patients with
surgically treated clear cell renal cell carcinoma.
In: J Urol
170 (2003), Nr. 6 Pt 1, S. 2225–32
Gold u.a. 1996 GOLD, P. J. ; FEFER, A. ;
THOMPSON, J. A.:
Paraneoplastic manifestations of renal cell carcinoma.
In: Semin Urol Oncol
14 (1996), Nr. 4, S. 216–22
Heng, D. Y. C.; Wells, J. C.; Rini, B. I.; Beuselinck,
B.; Lee, J.-L.; Knox, J. J.; Bjarnason, G. A.; Pal, S. K.;
Kollmannsberger, C. K.; Yuasa, T.; Srinivas, S.; Donskov, F.; Bamias, A.;
Wood, L. A.; Ernst, D. S.; Agarwal, N.; Vaishampayan, U. N.; Rha, S. Y.;
Kim, J. J. & Choueiri, T. K.
Cytoreductive nephrectomy in patients
with synchronous metastases from renal cell carcinoma: results from the
international metastatic renal cell carcinoma database consortium.
Eur Urol 2014,
66, 704-710.
G. Hudes, M. Carducci, P. Tomczak, J. Dutcher, R. Figlin, A. Kapoor,
E. Staroslawska, J. Sosman, D. McDermott, I. Bodrogi, Z. Kovacevic,
V. Lesovoy, I. G. H. Schmidt-Wolf, O. Barbarash, E. Gokmen, T. O’Toole,
S. Lustgarten, L. Moore, R. J. Motzer, and G. A. Trial.
Temsirolimus, interferon alfa, or both for advanced renal-cell
carcinoma.
N Engl J Med, 356 (22): 2271–2281, May 2007.
E. Jonasch et al., “Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease.,” NEJM, vol. 385, no. 22, pp. 2036–2046, 2021, doi: 10.1056/NEJMoa2103425.
Kattan u.a. 2001 KATTAN, M. W. ; REUTER, V. ; MOTZER, R. J. ; KATZ, J. ; RUSSO, P.: A postoperative prognostic nomogram for renal cell carcinoma.In: J Urol
166 (2001), Nr. 1, S. 63–7
Rana R. McKay u.a. Impact of angiotensin system inhibitors (ASI) on outcomes in patients (pts) with metastatic renal cell carcinoma (mRCC): Results from a pooled clinical trials database. J Clin Oncol 32, 2014 (suppl 4; abstr 437).
B. C. Leibovich, M. L. Blute, J. C. Cheville, C. M. Lohse, I. Frank, E. D.
Kwon, A. L. Weaver, A. S. Parker, und H. Zincke.
Prediction of progression after radical nephrectomy for patients with
clear cell renal cell carcinoma: a stratification tool for prospective
clinical trials.
Cancer, 97 (7): 1663–1671, Apr 2003.
doi: rm10.1002/cncr.11234.
URL https://dx.doi.org/10.1002/cncr.11234.
Ljungberg, B.; Albiges, L.; Bensalah, K.; Bex, A.;
Giles, R.; Hora, M.; Kuczyk, M.; Lam, T.; L.Marconi; Merseburger, A.;
Powles, T.; Staehler, M. & Volpe, A.
EAU Guidelines on Renal Cell Carcinoma
2018. https://uroweb.org/guidelines/renal-cell-carcinoma/
Mancuso und Sternberg 2005 MANCUSO, A. ;
STERNBERG, C. N.:
What’s new in the treatment of metastatic kidney cancer?
In: BJU Int
95 (2005), Nr. 9, S. 1171–80
Mickisch, G. H.; Garin, A.; van Poppel, H.; de Prijck,
L.; Sylvester, R.; , E. O. f. R. & of Cancer (EORTC) Genitourinary Group,
T.
Radical nephrectomy plus interferon-alfa-based immunotherapy
compared with interferon alfa alone in metastatic renal-cell carcinoma: a
randomised trial.
Lancet, 2001, 358, 966-970.
Motzer und Bukowski 2006 MOTZER, Robert J. ;
BUKOWSKI, Ronald M.:
Targeted therapy for metastatic renal cell carcinoma.
In: J Clin Oncol
24 (2006), Dec, Nr. 35, S. 5601–5608. -
URL https://dx.doi.org/10.1200/JCO.2006.08.5415
Motzer u.a. 2007 MOTZER, Robert J. ; HUTSON,
Thomas E. ; TOMCZAK, Piotr ; MICHAELSON, M. D. ;
BUKOWSKI, Ronald M. ; RIXE, Olivier ; OUDARD,
Stéphane ; NEGRIER, Sylvie ; SZCZYLIK, Cezary ;
KIM, Sindy T. ; CHEN, Isan ; BYCOTT, Paul W. ;
BAUM, Charles M. ; FIGLIN, Robert A.:
Sunitinib versus interferon alfa in metastatic renal-cell carcinoma.
In: N Engl J Med
356 (2007), Jan, Nr. 2, S. 115–124. -
URL https://dx.doi.org/10.1056/NEJMoa065044
Motzer, R. J.; Escudier, B.; McDermott, D. F.; George,
S.; Hammers, H. J.; Srinivas, S.; Tykodi, S. S.; Sosman, J. A.; Procopio,
G.; Plimack, E. R.; Castellano, D.; Choueiri, T. K.; Gurney, H.; Donskov,
F.; Bono, P.; Wagstaff, J.; Gauler, T. C.; Ueda, T.; Tomita, Y.; Schutz,
F. A.; Kollmannsberger, C.; Larkin, J.; Ravaud, A.; Simon, J. S.; Xu,
L.-A.; Waxman, I. M.; Sharma, P. & CheckMate025
Nivolumab versus
Everolimus in Advanced Renal-Cell Carcinoma.
New Engl J Med 2015, 373,
1803-1813.
Motzer, R. J.; Hutson, T. E.; Glen, H.;
Michaelson, M. D.; Molina, A.; Eisen, T.; Jassem, J.; Zolnierek, J.;
Maroto, J. P.; Mellado, B.; Melichar, B.; Tomasek, J.; Kremer, A.; Kim,
H.-J.; Wood, K.; Dutcus, C. & Larkin, J.
Lenvatinib, everolimus,
and the combination in patients with metastatic renal cell carcinoma: a
randomised, phase 2, open-label, multicentre trial.
The Lancet.
Oncology, 2015a, 16, 1473-1482.
R. J. Motzer, B. Escudier, S. Oudard, T. E. Hutson, C. Porta, S. Bracarda,
V. Gruenwald, J. A. Thompson, R. A. Figlin, N. Hollaender, G. Urbanowitz,
W. J. Berg, A. Kay, D. Lebwohl, und A. Ravaud.
Efficacy of everolimus in advanced renal cell carcinoma: a
double-blind, randomised, placebo-controlled phase iii trial.
Lancet, Jul 2008.
R. J. Motzer et al.“Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial.,” Journal of clinical oncology : official journal of the American Society of Clinical Oncology, vol. 31, no. 30, pp. 3791–3799, 2013.
S. Negrier, D. Perol, A. Ravaud, C. Chevreau, J. O. Bay, R. Delva, E. Sevin,
A. Caty, und B. Escudier.
Medroxyprogesterone, interferon alfa-2a, interleukin 2, or
combination of both cytokines in patients with metastatic renal carcinoma of
intermediate prognosis: results of a randomized controlled trial.
Cancer, 110 (11): 2468–2477, Dec 2007.
Powles, T.; Albiges, L.; Staehler, M.; Bensalah,
K.; Dabestani, S.; Giles, R. H.; Hofmann, F.; Hora, M.; Kuczyk, M. A.;
Lam, T. B.; Marconi, L.; Merseburger, A. S.; Fernández-Pello, S.; Tahbaz,
R.; Volpe, A.; Ljungberg, B. & Bex, A.
Updated European Association
of Urology Guidelines Recommendations for the Treatment of First-line
Metastatic Clear Cell Renal Cancer.
European urology, 2017
Rini, B. I.; Escudier, B.; Tomczak, P.; Kaprin, A.;
Szczylik, C.; Hutson, T. E.; Michaelson, M. D.; Gorbunova, V. A.; Gore, M.
E.; Rusakov, I. G.; Negrier, S.; Ou, Y.; Castellano, D.; Lim, H. Y.;
Uemura, H.; Tarazi, J.; Cella, D.; Chen, C.; Rosbrook, B.; Kim, S. &
Motzer, R. J.
Comparative effectiveness of axitinib versus sorafenib in
advanced renal cell carcinoma (AXIS): a randomised phase 3 trial.
Lancet,
2011, 378, 1931-1939.
Sternberg, C. N.; Davis, I. D.; Mardiak, J.; Szczylik,
C.; Lee, E.; Wagstaff, J.; Barrios, C. H.; Salman, P.; Gladkov, O. A.;
Kavina, A.; Zarbá, J. J.; Chen, M.; McCann, L.; Pandite, L.; Roychowdhury,
D. F. & Hawkins, R. E.
Pazopanib in locally advanced or metastatic
renal cell carcinoma: results of a randomized phase III trial.
2010,
28, 1061-1068.
Weight, C. J.; Larson, B. T.; Fergany, A. F.; Gao, T.; Lane, B. R.; Campbell, S. C.; Kaouk, J. H.; Klein, E. A. & Novick, A. C.
Nephrectomy induced chronic renal insufficiency is associated with increased risk of
cardiovascular death and death from any cause in patients with localized
cT1b renal masses.
J Urol, 2010, 183, 1317-1323
Zini, L.; Perrotte, P.; Capitanio, U.; Jeldres, C.; Shariat, S. F.; Antebi, E.; Saad, F.; Patard, J.; Montorsi, F. &
Karakiewicz, P. I.
Radical versus partial nephrectomy: effect on
overall and noncancer mortality.
Cancer, 2009, 115,
1465-1471
Zisman u.a. 2002 ZISMAN, A. ; PANTUCK, A. J. ;
WIEDER, J. ; CHAO, D. H. ; DOREY, F. ;
SAID, J. W. ; DEKERNION, J. B. ; FIGLIN, R. A. ;
BELLDEGRUN, A. S.:
Risk group assessment and clinical outcome algorithm to predict the
natural history of patients with surgically resected renal cell carcinoma.
In: J Clin Oncol
20 (2002), Nr. 23, S. 4559–66
 Deutsche Version: Nierenzellkarzinom
Deutsche Version: Nierenzellkarzinom