You are here: Urology Textbook > Ureters > Upper tract urothelial cancer
Upper Tract Urothelial Cancer: TNM Staging, Diagnosis, and Treatment
Upper tract urothelial carcinomas (UTUC) are malignant tumors of the urothelium of the ureter, renal pelvis, or calyces with similar properties to bladder carcinoma concerning etiology and pathology. EAU guidelines UTUC. Synonym: transitional cell carcinoma of the upper tract.
 |
Epidemiology
- Peak incidence 70–80 years of age, incidence 2/100000.
- Female to male ratio = 1:2 to 1:3.
- 5–10% of all urothelial cancers arise from the upper urinary tract.
- 2–4% of patients with bladder carcinoma experience a recurrence in the upper urinary tract. 30% of patients after cystectomy suffer a relapse in the upper urinary tract within 15 years. The incidence of UTUC is rising due to the effective treatment of bladder carcinoma with better survival rates (length time bias).
Etiology of Upper Tract Urothelial Carcinoma
See etiology of bladder carcinoma.
TNM Tumor Stages of UTUC
T: Stage of the primary tumor.
- Ta: Non-invasive papillary carcinoma.
- Tis: Carcinoma in situ.
- T1: Tumor invades subepithelial connective tissue.
- T2: Tumor invades muscular layer.
- T3: Tumor invades the periureteric or peripelvic fat or the renal parenchyma.
- T4: Tumor invades adjacent organs or through the kidney into perinephric fat.
N: Involvement of regional lymph nodes.
- N0: No regional lymph node metastasis.
- N1: Metastasis in a single lymph node 2 cm or less in the greatest dimensions.
- N2: Single lymph node metastasis greater than 2 cm or multiple lymph node metastasis.
M: Distant metastasis.
- M0: No distant metastasis.
- M1: Distant metastasis present.
G: Grading.
- Urothelial papilloma.
- Papillary urothelial neoplasm of low malignant potential (PUNLMP).
- Low-grade urothelial carcinoma.
- High-grade urothelial carcinoma.
Pathology of Upper Tract Urothelial Carcinoma
Histology:
Upper tract urothelial carcinoma is urothelial carcinoma in 95%, but squamous cell carcinoma or adenocarcinoma is rarely possible.
Growth pattern:
Papillary morphology with early invasion into deeper layers compared to bladder carcinoma [fig. UTUC of the renal pelvis]. 50% of papillary tumors of the renal pelvis are already staged T1 or T2. A multifocal growth pattern is found in up to 90% of the specimens after nephroureterectomy.
Metastasis:
Lymphatic spread of proximal tumors into para-aortic, paracaval, and hilar lymph nodes. Tumors of the middle third of the ureter spread into the iliac, para-aortic, and paracaval lymph nodes, and tumors of the distal ureter spread into the obturator and iliac lymph nodes. Hematogenous metastases are found in the lungs, liver, bone, and adrenal glands.
Signs and Symptoms of Upper Tract Urothelial Cancer
- Hematuria, worm-like clots
- Dysuria, flank pain, renal colic
- Hydronephrosis Symptoms of advanced tumor disease (B symptoms)
Diagnosis of Upper Tract Urothelial Cancer
Urine cytology:
The microscopic examination of exfoliated urothelial cells in voided urine or an upper urinary tract irrigation sample can reliably identify high-grade tumor cells. Well-differentiated tumors are less likely to exfoliate cells, and the distinction to inflammatory changes is unreliable, see section urine cytology.
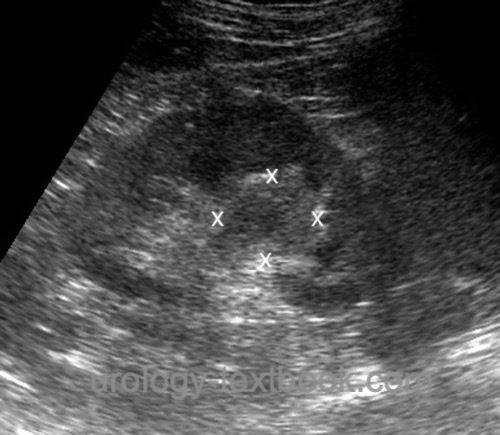
Ultrasound imaging:
Renal ultrasound: Hydronephrosis? Hypoechoic mass in the renal sinus [fig. Ultrasound imaging of UTUC]? Bladder ultrasound: Bladder tumor? Abdominal ultrasound: Metastasis of the liver?
 |
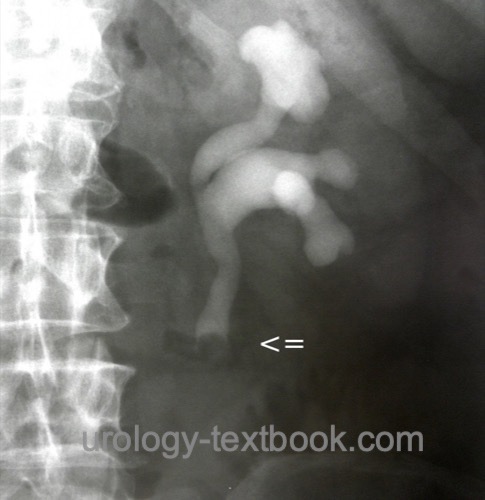
Retrograde pyelography:
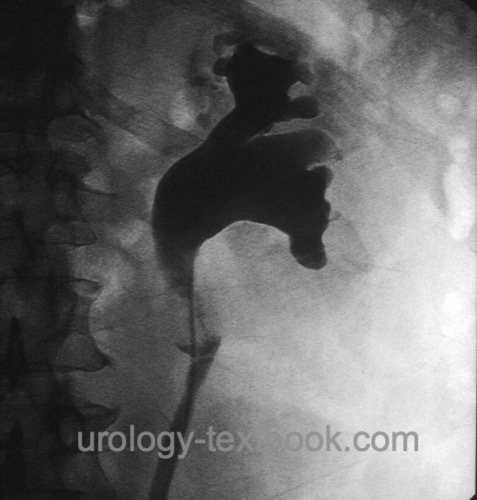
If irrigation fluid for urine cytology is needed, it must be collected before administering the high osmolar contrast medium. Signs of upper tract urothelial cancer in retrograde pyelography are ureteral strictures, filling defects or missing contrast in the calyces [figure retrograde pyelography of UTUC].
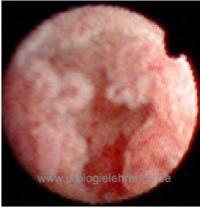
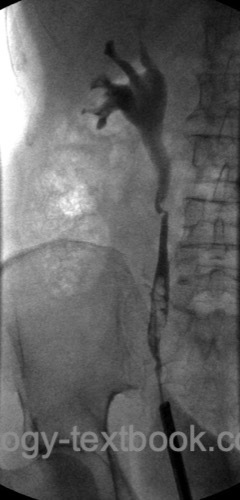
Ureterorenoscopy:
Rigid or flexible ureteroscopy with multiple biopsies of suspicious lesions [fig. ureteroscopy of UTUC].
 |
Cystoscopy:
Cystoscopy is done at the initial diagnosis of UTUC and during follow-up to rule out urothelial carcinoma of the lower urinary tract.
Staging of UTUC:
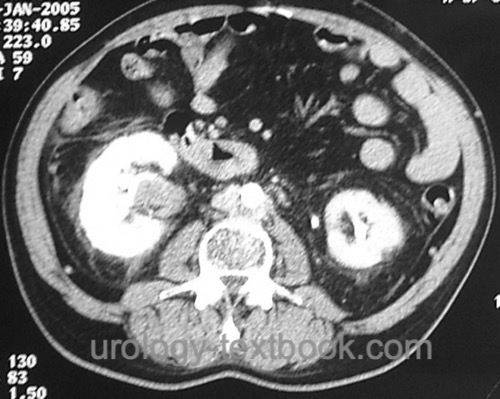
All patients need an abdominal contrast-enhancing CT scan [fig. CT 1 of UTUC and CT 2 of UTUC] and chest CT. Initiate a bone scintigraphy for patients with skeletal pain or an advanced tumor stage.
 |
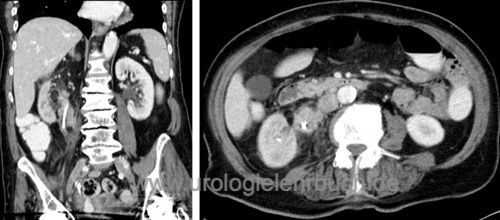 |
Intravenous urography:
Intravenous urography is used to diagnose UTUC, but it is insufficient for staging and cannot replace imaging with computed tomography. Signs of UTUC are ureteral stricture, filling defects, hydronephrosis, or missing contrast on the affected side [fig. IVP of UTUC].
 |
Risk Stratification of Non-Metastatic UTUC
The EAU guidelines differentiate between low-risk and high-risk non-metastatic UTUC to identify those patients who are more likely to benefit from a less aggressive kidney-sparing treatment approach:
Low-risk UTUC:
All factors must apply: unifocal tumor below 2 cm, low-grade pathology or cytology, and non-invasive tumor in CT.
High-risk UTUC:
With any of these factors: hydronephrosis, tumor size >2 cm, high-grade pathology or cytology, multifocal disease, status after cystectomy for high-grade bladder carcinoma, or variant histology.
Treatment of Low-Risk Non-Metastatic UTUC
Kidney-sparing surgery:
First-choice for low-risk tumors if technically feasible. For high-risk tumors, a treatment option if the preservation of kidney function is mandatory. The high recurrence rate of high-grade tumors is problematic [fig. recurrence of UTUC].
 |
Surgical therapy of distal UTUC:
The surgical therapy of distal UTUC includes distal ureteral resection and ureteroneocystostomy with psoas hitch or Boari flap.
UTUC in the mid-third of the ureter:
Ureteral segment resection and ureteroureterostomy.
UTUC in the proximal ureter and renal pelvis:
Kidney-sparing is technically challenging and not often performed: pyelotomy and open tumor resection, partial resection of the renal pelvis, or partial nephrectomy with, e.g., upper pole resection.
Multifocal ureteral tumors:
Ureteral resection with ileum interposition is possible in selected cases.
Endoscopic treatment options:
Retrograde or antegrade approach to the upper urinary tract: endoscopic ablation or tumor destruction with laser or electrocautery (Chew et al., 2005) (Ho et al., 2005).
Adjuvant instillation therapy of the upper urinary tract:
Adjuvant upper urinary tract instillation therapy is possible with BCG or mitomycin C via a nephrostomy or ureteral stent. The effectiveness is controversial.
Postoperative instillation therapy of the bladder:
A single postoperative bladder instillation after nephroureterectomy with mitomycin C reduces the risk of a recurrence in the urinary bladder (OBrien et al., 2011). By analogy, this is also an option after kidney-preserving interventions. Extravasation should be ruled out with a cystography before instillation.
Treatment of High-Risk Non-Metastatic UTUC
Neoadjuvant Chemotherapy:
In retrospective studies and in analogy to bladder carcinoma, advantages for neoadjuvant chemotherapy have been published. Neoadjuvant chemotherapy is advantageous considering the limited possibility for chemotherapy containing cisplatin after nephroureterectomy (Porten et al., 2014). Neoadjuvant or adjuvant chemotherapy should be strongly considered for T3–4 tumor stage or suspected lymph node metastases. See the section on chemotherapy of metastatic bladder carcinoma for dosage and results.
Adjuvant Chemotherapy:
A randomized study was able to demonstrate clear advantages for adjuvant chemotherapy after nephroureterectomy in patients with pT2–4 or pN+: 71% vs. 46% progression-free survival after three years. Patients with adequate renal function received cisplatin and gemcitabine, while carboplatin was administered to patients with impaired renal function (Birtle et al., 2020).
Radical nephroureterectomy:
Radical nephroureterectomy is the treatment of the first choice for high-risk non-metastatic UTUC. Consider kidney-sparing surgery for patients with chronic kidney disease (see above). Principle steps of radical nephroureterectomy are radical nephrectomy, complete wide resection of the ureter with a bladder cuff, and lymphadenectomy.
Surgical approaches:
A variety of surgical approaches are possible: retroperitoneal abdominal incision from subcostal to suprapubic, two extraperitoneal incisions (flank incision and e.g., Gibson or Pfannenstiel), transperitoneal midline laparotomy or laparoscopic (robotic-assisted) nephroureterectomy (Matin et al., 2005). In retrospective comparisons, the laparoscopic approach has a lower complication rate and shorter hospital stay (Hanna et al., 2012). A randomized study of advanced tumors (T3–4) and high-grade tumors showed fewer recurrences and metastases with the open surgical approach (Simone et al., 2009). See the section nephroureterectomy for details of the surgical techniques.
Lymphadenectomy for UTUC:
Lymphadenectomy is done in the lymph drainage area of the tumor: aortal, interaortocaval, or paracaval lymphadenectomy for tumors of the proximal and mid-third of the ureter, and pelvic lymphadenectomy for tumors of the distal ureter. Lymphadenectomy improves the prognosis in retrospective trials, but the extent of dissection remains controversial (Lenis et al., 2018).
Postoperative instillation therapy of the bladder:
A single postoperative bladder instillation with mitomycin C reduces the risk of a recurrence (17% versus 27%) in the urinary bladder (OBrien et al., 2011).
Chemotherapy and immunotherapy for metastatic UTUC:
See the section on chemotherapy of metastatic bladder carcinoma for dosage and results.
Follow-up of Upper Tract Urothelial Cancer
Follow-up examinations are performed for at least five years to diagnose metachronous bladder carcinoma, local recurrence, or distant metastases. The EAU guidelines differentiate between low-risk and high-risk UTUC; there is a high risk of recurrence in the presence of any risk factors: tumor size >2 cm, high-grade pathology, multifocal manifestation, and UTUC recurrence after cystectomy for bladder cancer.
Follow-up after nephroureterectomy for low-risk UTUC:
Cystoscopy after three months and then yearly. Contrast-enhanced CT of the abdomen and chest yearly.
Follow-up after nephroureterectomy for high-risk UTUC:
Cystoscopy every three months for two years, every six months after that until five years, and then yearly. Contrast-enhanced CT of the abdomen and chest every six months for two years and then yearly.
Follow-up after kidney-sparing management:
Cystoscopy, urine cytology and contrast-enhanced CT of abdomen after three month, six month and than yearly. Chest CT, ureteroscopy and irrigation urine cytology of the upper tract individually depending on risk and imaging results.
Prognosis of Upper Tract Urothelial Carcinoma
5-years survival depending on tumor stage:
T1 60–90%, T2 43–75%, T3 16–33%, T4 or lymph node metastasis or distant metastasis under 5%.
5-years survival depending on grading:
40–87% for G1–2 tumors, 0–33% for G3 tumors.
| Ureteritis cystica | Index | Nephroureterectomy |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
EAU guidelines: Upper Tract Urothelial Carcinoma
A. Birtle et al., “Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial.,” Lancet, vol. 395, no. 10232, pp. 1268–1277, 2020, doi: 10.1016/S0140-6736(20)30415-3.
Browne u.a. 2005 BROWNE, R. F. ; MEEHAN,
C. P. ; COLVILLE, J. ; POWER, R. ; TORREGGIANI,
W. C.:
Transitional cell carcinoma of the upper urinary tract: spectrum of
imaging findings.
In: Radiographics
25 (2005), Nr. 6, S. 1609–27
Chew u.a. 2005 CHEW, B. H. ; PAUTLER, S. E. ;
DENSTEDT, J. D.:
Percutaneous management of upper-tract transitional cell carcinoma.
In: J Endourol
19 (2005), Nr. 6, S. 658–63
Hanna, N.; Sun, M.; Trinh, Q.; Hansen, J.; Bianchi, M.;
Montorsi, F.; Shariat, S. F.; Graefen, M.; Perrotte, P. & Karakiewicz, P.
I.
Propensity-score-matched comparison of perioperative outcomes
between open and laparoscopic nephroureterectomy: a national series.
Eur
Urol, 2012, 61, 715-721.
Ho und Chow 2005 HO, K. L. ; CHOW, G. K.:
Ureteroscopic resection of upper-tract transitional-cell carcinoma.
In: J Endourol
19 (2005), Nr. 7, S. 841–8
Matin 2005 MATIN, S. F.:
Radical laparoscopic nephroureterectomy for upper urinary tract
transitional cell carcinoma: current status.
In: BJU Int
95 Suppl 2 (2005), S. 68–74
S. Porten et al., “Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma.,” Cancer, vol. 120, no. 12, pp. 1794–1799, 2014, doi: 10.1002/cncr.28655.
O'Brien, T.; Ray, E.; Singh, R.; Coker, B.; Beard, R. &
of Urological Surgeons Section of Oncology, B. A.
Prevention of bladder
tumours after nephroureterectomy for primary upper urinary tract
urothelial carcinoma: a prospective, multicentre, randomised clinical
trial of a single postoperative intravesical dose of mitomycin C (the
ODMIT-C Trial).
Eur Urol, 2011, 60, 703-710.
Tawfiek und Bagley 1997 TAWFIEK, E. R. ; BAGLEY,
D. H.:
Upper-tract transitional cell carcinoma.
In: Urology
50 (1997), Nr. 3, S. 321–9
 Deutsche Version: Harnleiterkarzinom und Nierenbeckenkarzinom und Diagnose und Therapie des Urothelkarzinom des oberen Harntrakts
Deutsche Version: Harnleiterkarzinom und Nierenbeckenkarzinom und Diagnose und Therapie des Urothelkarzinom des oberen Harntrakts
Urology-Textbook.com – Choose the Ad-Free, Professional Resource
This website is designed for physicians and medical professionals. It presents diseases of the genital organs through detailed text and images. Some content may not be suitable for children or sensitive readers. Many illustrations are available exclusively to Steady members. Are you a physician and interested in supporting this project? Join Steady to unlock full access to all images and enjoy an ad-free experience. Try it free for 7 days—no obligation.
New release: The first edition of the Urology Textbook as an e-book—ideal for offline reading and quick reference. With over 1300 pages and hundreds of illustrations, it’s the perfect companion for residents and medical students. After your 7-day trial has ended, you will receive a download link for your exclusive e-book.