You are here: Urology Textbook > Surgery (procedures) > Robotic-assisted radical prostatectomy
Surgical Steps and Complications of Robotic-Assisted Radical Prostatectomy
Indications for Robotic-Assisted Radical Prostatectomy
Radical prostatectomy (RPE) is the gold standard of curative therapy in patients with localized prostate cancer and a life expectancy of at least 10 years. For a detailed description of the surgical treatment indications and results, see:
Contraindications
Absolute contraindications are uncorrected coagulation disorders and untreated urinary tract infections. Further contraindications depend on the surgical risk due to the comorbidity of the patient and the impact of prostatectomy on the life expectancy of the patient. Specific relative contraindications to laparoscopic prostatectomy include (depending on expertise) prior complex pelvic surgery, morbid obesity, very large prostate glands, and after pelvic radiation therapy (e.g., for prostate cancer).
Surgical Technique (Step by Step) of Laparoscopic (Robotic-Assisted) Radical Prostatectomy
Preoperative Patient Preparation
Timing of surgery:
A nerve-sparing prostatectomy should be performed no earlier than eight weeks after prostate biopsy and three months after TURP. The interval leads to reduced adhesions between the prostate and neurovascular bundle.
Bowel preparation:
The day before surgery: clear liquid diet and an enema before sleeping.
Perioperative antibiotic prophylaxis:
Before skin incision, e.g., 2nd generation cephalosporin. See also section perioperative antibiotic prophylaxis.
Patient positioning:
Supine position with slight lumbar hyperextension. Secure fixation should be achieved with belts over the chest, pelvis, and calves, with the arms resting next to the body, and a steep Trendelenburg positioning should be possible. Disinfection and sterile draping of the abdomen.
Anesthesia:
General anesthesia.
Surgical Approach Via Laparoscopy
The following description of laparoscopic transperitoneal prostatectomy was modified from (Tuerk et al., 2001) and (Guillonneau et al., 2000a). Technical alternatives are extraperitoneal endoscopic prostatectomy (Stolzenburg et al., 2007) and robotic-assisted laparoscopic or endoscopic prostatectomy (Zorn et al., 2009) (Coelho et al., 2010). All mentioned techniques differ in details regarding the access, but the dissection boundaries at the prostate are the same.
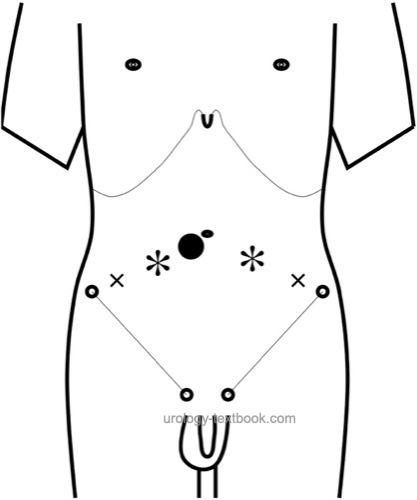
Trocar Positions:
Place the camera port just below the umbilicus, either with the Veress needle or with an open technique. Move the patient in Trendelenburg positioning after the creation of a pneumoperitoneum. Place two ports with 10 mm diameter left and right latero-distal of the umbilicus and two 5 mm ports left and right lateral of the 10 mm ports. Beware of the epigastric vessels.
 |
Pelvic Lymphadenectomy:
See the section laparoscopic pelvic lymph node dissection.
Dissection of the Seminal Vesicles:
Incise the peritoneum along the course of the vas deferens into the Douglas space on both sides. Unite the incision with the opposite side in the midline deep in the pelvis. Transect both vas below the iliac vessels and mobilize them to the seminal vesicles. Incise the surrounding connective tissue of the seminal vesicles and dissect the seminal vesicles. Use clips for the blood supply radiating in from the lateral side. Let the assistant pull the prostate cranially with the vas deferens, and incise the stretched Denovillier fascia just below the seminal vesicles. Dissect bluntly the prostate from the rectum with a dissecting swab.
Ventral Prostate Dissection:
Fill the bladder with 200 ml saline and incise the parietal peritoneum around the bladder. Transect the urachus as cranially as possible. Extend the peritoneal incision on both sides of the bladder to the medial umbilical fold. Find the retrovesical space and detach the bladder from the abdominal wall. Continue with the dissection caudally until reaching the endopelvic fascia and the prostate.
Empty the bladder and use an endoretractor to displace the bladder cranially. Cleanse the prostate from covering fatty tissue, coagulate, and transect interfering superficial veins. Coagulate and transect the puboprostatic ligament after incision of the endopelvic fascia. Bluntly expose the apical area of the prostate with a dissecting swab. The dorsal venous plexus is secured with a suture ligation (Vicryl 1-0).
Transection of the Bladder Neck:
The border between the bladder and the prostate becomes visible with traction on the catheter with an inflated balloon. Incise the bladder neck horizontally using bipolar coagulation or a harmonic scalpel. After opening the bladder, unblock the catheter and let the assistant grasp and lift the catheter anteriorly to provide traction on the prostate and bladder neck.
Continue the transection of the bladder neck laterally as far as the anatomical plane is clearly visible. Incise the posterior bladder neck after identification of the ureteric orifices. A large median lobe may complicate this step. Continue the dissection posteriorly until the already dissected seminal vesicles and vas deferens become visible. The assistant grasps the vas deferens and pulls them toward the symphysis; this leads to tension on the prostatic pedicles.
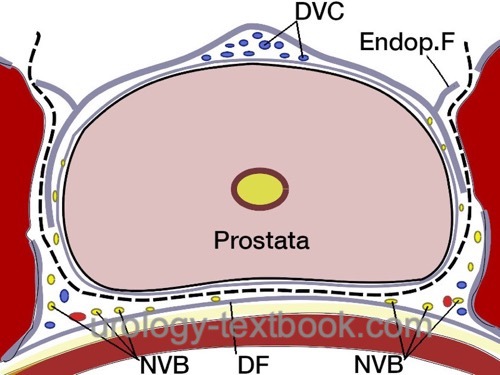
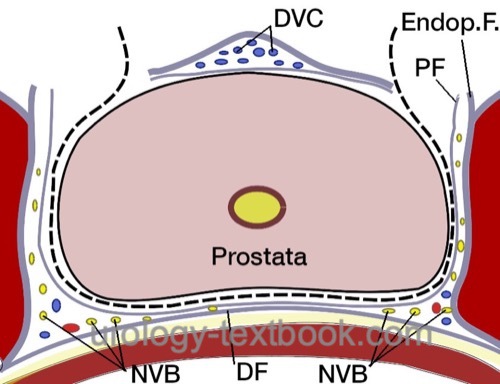
Nervesparing and Transection of the Prostatic Pedicles:
Transect the prostate pedicles step by step between clips or with a harmonic scalpel. The direction of transection of the prostatic pedicles is initially strictly lateral along the bladder neck. The fascia overlying the prostate is opened to isolate the neurovascular bundle. Further transection of the prostatic pedicles is done tangentially along the neurovascular bundle. Depending on the patient's wishes and tumor stage, nerve-sparing can be performed to a varying extent; see the following figures:
 |
 |
Division of the Urethra:
Transect the dorsal vein complex tangentially to the ventral prostate surface with sufficient distance from the suture ligation. The dissection should lead to a good visualization of the urethra and apex to enable maximal preservation of urethral length and to avoid injury to the neurovascular bundles. Divide the ventral urethra with cold scissors; the posterior and dorsal sphincters are divided after the removal of the catheter. Store the prostate in the iliac fossa using a specimen bag.
Vesicourethral Anastomosis:
The reconstruction of the dorsal tissue structures (dorsal sphincter fibers with the Denovilliers fascia) before the actual anastomosis should improve the early continence and tightness of the anastomosis (Rocco et al., 2009). In the initial description, the anastomosis was performed with interrupted sutures. The vesicourethral anastomosis is easier and faster with a running double-armed barbed monofilament suture. The suture starts posteriorly and is continued on both sides in the anterior direction. Before completion of the anastomosis, a 20 CH catheter is inserted and serves as a guide for the final stitches. If the bladder neck is wide, the bladder neck is closed anteriorly ("tennis racket" suture). Irrigate the bladder after anastomosis to eliminate clots and to assess for urinary leaks. Carefully check for bleeding.
Organ Retrieval and Wound Closure:
Extract the specimen bag through the umbilical port site after incision of the rectus sheath. Place a pelvic drain (e.g., closed gravity system) via a 5 mm port site. Close the fascia at the organ retrieval site and the other 10 mm port site (USP 1). Skin sutures.
Technical modifications of laparoscopic radical prostatectomy:
The following modifications of the standard technique are used (also in combination):
- Robot-assisted laparoscopy: the three-dimensional view, articulated instruments with several degrees of freedom, a filtered hand tremor and the ergonomic working position simplify laparoscopic surgery. Robotic-assisted prostatectomy became the standard procedure for radical prostatectomy in many parts of the world.
- Extraperitoneal endoscopic prostatectomy: avoiding the transperitoneal dissection reduces the risk of complications like bowel injury and postoperative ileus. It is advantageous in obese patients and after prior abdominal surgery.
- Single-port laparoscopy: the surgery is performed via a trocar with multiple channels and with special angulated instruments. There is also a single-port platform available for robot-assisted laparoscopy (Dobbs et al., 2020).
- Transvesical endoscopic prostatectomy: with a robot-assisted single-post platform (Kaouk et al., 2021).
Postoperative Care
- General measures: consider patient-controlled analgesia for pain management. Early mobilization and exercises to prevent thrombosis and pneumonia. Thrombosis prophylaxis. Laboratory tests (hemoglobin, creatinine), regular physical examination of the abdomen, and incision wound.
- Diet advancement: clear liquid diet on day one, soft food diet the following days until bowel movement.
- Drains and catheters: keep the pelvic drain for 1–2 days. In routine cases, the catheter stays for seven days and cystography before removal is optional. Depending on the extent of bladder neck resection and reconstruction, the catheter stay should be extended to 10–14 days before first cystography.
Complications
In principle, the same complications can be expected as with retropubic radical prostatectomy. The laparoscopic robotic-assisted procedure has advantages in terms of blood loss, length of stay, catheter duration and return to normal activity. In large comparative studies, no or no relevant differences were found in terms of potency, continence, or oncologic cure (DellOglio et al., 2020). The prospective-controlled LAPPRO study found no relevant differences for developing hernias (7.3–8.4% hernia risk) after open-surgical or robotic-assisted laparoscopic prostatectomy (Nilsson et al., 2022).
| Laparoscopic pelvic lymphadenectomy | Index | Perineal radical prostatectomy |
Index: 1–9 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z
References
P. Dell’Oglio, A. Mottrie, and E. Mazzone, “Robot-assisted radical prostatectomy vs. open radical prostatectomy: latest evidences on perioperative, functional and oncological outcomes.,” Current opinion in urology, vol. 30, no. 1, pp. 73–78, 2020, doi: 10.1097/MOU.0000000000000688.
Guillonneau und Vallancien 2000a
GUILLONNEAU, B. ; VALLANCIEN, G.:
Laparoscopic radical prostatectomy: the Montsouris experience.
In: J Urol
163 (2000), Feb, Nr. 2, S. 418–422
Guillonneau und Vallancien 2000b
GUILLONNEAU, B. ; VALLANCIEN, G.:
Laparoscopic radical prostatectomy: the Montsouris technique.
In: J Urol
163 (2000), Jun, Nr. 6, S. 1643–1649
Kaouk J, Beksac AT, Abou Zeinab M, Duncan A, Schwen ZR, Eltemamy M. Single Port Transvesical Robotic Radical Prostatectomy: Initial Clinical Experience and Description of Technique. Urology. 2021 Sep;155:130-137. doi: 10.1016/j.urology.2021.05.022
H. Nilsson et al., “Risk of hernia formation after radical prostatectomy: a comparison between open and robot-assisted laparoscopic radical prostatectomy within the prospectively controlled LAPPRO trial.,” Hernia, vol. 26, no. 1, pp. 157–164, 2022, doi: 10.1007/s10029-020-02178-7.
Rocco, F. & Rocco, B.
Anatomical reconstruction
of the rhabdosphincter after radical prostatectomy.
BJU Int, 2009,
104, 274-281.
J. A. Smith, S. S. Howards, G. M. Preminger, and R. R. Dmochowski, Hinman’s Atlas of Urologic Surgery Revised Reprint. Elsevier, 2019.
Stolzenburg, J.; Rabenalt, R.; Do, M.; Truss, M. C.;
Burchardt, M.; Herrmann, T. R.; Schwalenberg, T.; Kallidonis, P. &
Liatsikos, E. N.
Endoscopic extraperitoneal radical prostatectomy: the
University of Leipzig experience of 1,300 cases.
World J Urol, 2007,
25, 45-51.
Türk u.a. 2001 TüRK, I. ; DEGER, S. ;
WINKELMANN, B. ; SCHöNBERGER, B. ; LOENING,
S. A.:
Laparoscopic radical prostatectomy. Technical aspects and experience
with 125 cases.
In: Eur Urol
40 (2001), Jul, Nr. 1, S. 46–52; discussion 53
Zorn, K. C.; Gautam, G.; Shalhav, A. L.; Clayman, R. V.; Ahlering, T. E.; Albala, D. M.; Lee, D. I.; Sundaram, C. P.; Matin, S.
F.; Castle, E. P.; Winfield, H. N.; Gettman, M. T.; Lee, B. R.; Thomas,
R.; Patel, V. R.; Leveillee, R. J.; Wong, C.; Badlani, G. H.; Rha, K. H.;
Eggener, S. E.; Wiklund, P.; Mottrie, A.; Atug, F.; Kural, A. R.; Joseph,
J. V. & of the Society of Urologic Robotic Surgeons, M.
Training,
credentialing, proctoring and medicolegal risks of robotic urological
surgery: recommendations of the society of urologic robotic surgeons.
J
Urol, 2009, 182, 1126-1132.
 Deutsche Version: Laparoskopische (robotisch-assistierte) radikale Prostatektomie
Deutsche Version: Laparoskopische (robotisch-assistierte) radikale Prostatektomie